Abstract
Whole blood viscosity (WBV) is the intrinsic resistance to flow developed due to the frictional force between adjacent layers of flowing blood. Elevated WBV is an independent risk factor for stroke. Poor microcirculation due to elevated WBV can prevent adequate perfusion of the brain and might act as an important secondary factor for hypoperfusion in acute ischaemic stroke. In the present study, we examined the association of WBV with basal cerebral perfusion assessed by CT perfusion in acute ischaemic stroke. Confirmed acute ischemic stroke patients (n = 82) presenting in hours were recruited from the single centre. Patients underwent baseline multimodal CT (non-contrast CT, CT angiography and CT perfusion). Where clinically warranted, patients also underwent follow-up DWI. WBV was measured in duplicate within 2 h after sampling from 5-mL EDTA blood sample. WBV was significantly correlated with CT perfusion parameters such as perfusion lesion volume, ischemic core volume and mismatch ratio; DWI volume and baseline NIHSS. In a multivariate linear regression model, WBV significantly predicted acute perfusion lesion volume, core volume and mismatch ratio after adjusting for the effect of occlusion site and collateral status. Association of WBV with hypoperfusion (increased perfusion lesion volume, ischaemic core volume and mismatch ratio) suggest the role of erythrocyte rheology in cerebral haemodynamic of acute ischemic stroke. The present findings open new possibilities for therapeutic strategies targeting erythrocyte rheology to improve cerebral microcirculation in stroke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Whole blood viscosity (WBV) is the intrinsic resistance to flow developed due to the frictional force between adjacent layers of flowing blood. Elevated WBV reduces tissue perfusion in the affected organ [1,2,3]. For instance, it is associated with reduced coronary collateral circulation in patients with chronic total occlusion [4], and with increased myocardial infarct size and left ventricular dysfunction [2]. More recently, WBV is also shown to be an independent and significant predictor of early left ventricular apical thrombus formation complicating a first-ever anterior wall myocardial infarction [5]. In addition, elevated WBV is a risk factor of primary and secondary stroke [6,7,8], and possibly could be an important secondary factor determining cerebral reperfusion in acute ischemic stroke.
Cerebral blood vessels have an essential ability to auto-regulate their blood flow by altering their diameter maintaining perfusion with minimal changes to the flow dynamics. After an ischemic stroke however, the affected blood vessels exhaust their auto-regulatory capacity [9]. The reperfusion therapies, thrombolysis and endovascular thrombectomy, pursue vessel recanalization, but the time between stroke onset and recanalization can be delayed for many hours. This leads to progression of tissue infarction (core) and decreases the volume of salvageable tissue (penumbra). The rate at which the infarct grows varies widely [10, 11], and depends on several secondary variables such as collateral circulation [12, 13] and competent cerebral microcirculation [14].
Poor microcirculation due to elevated WBV can prevent adequate perfusion of the brain at the advent of failed auto-regulation and might act as an important secondary factor for hypoperfused tissue growth. In cerebral microvasculature, erythrocytes must traverse through blood vessel whose diameter is much smaller than their own (typically 7 µm). Therefore, to facilitate tissue perfusion, erythrocytes need to be able to deform and assume a parachute-like shape. In the state of elevated WBV, which involves both increased plasma viscosity and decreased erythrocyte deformability, this physiological process is inhibited leading to increased stenotic peripheral resistance and microcirculation sludging [15].
The definitive role of WBV in determining the perfusion lesion and hypoperfused tissue growth in acute ischemic stroke is unknown, but previous studies have suggested a role for elevated WBV in influencing cerebral perfusion, in general [16,17,18]. While these previous studies [16,17,18,19] do provide proof-of-concept regarding the reciprocal association of WBV and cerebral blood flow, they either did not use contemporary technique to quantify cerebral perfusion or did not measure the perfusion specifically in hypoperfused regions of the brain. In the present study, we aim to examine the association between WBV and cerebral perfusion in a cohort of confirmed acute ischemic stroke patients who underwent baseline multimodal computed tomography (mCT). We hypothesised that WBV will be associated with unfavourable cerebral perfusion parameters assessed by baseline CT perfusion (CTP) and follow-up diffusion-weighted (DW) magnetic resonance imaging (MRI) volume.
Materials and methods
Patients
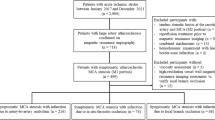
Consecutive ischemic stroke patients (clinico-radiological diagnosis) presenting in hours (8 am to 5 pm weekdays) at the John Hunter Hospital (Newcastle, Australia) between September 2018 and February 2020 were recruited. All patients underwent acute brain multimodal CT, consisting of brain non-contrast CT (NCCT), CT angiography (CTA) and CTP. When clinically warranted, patients also underwent follow-up brain diffusion-weighted (DWI) magnetic resonance imaging (MRI). Baseline demographics, imaging characteristics and stroke severity assessed using the National Institutes of Health Stroke Scale (NIHSS) were recorded. The study was approved by the Hunter New England Health District human research ethics committee (14/10/15/4.02).
Imaging
Patients were scanned using a Toshiba Aquilion (Tokyo, Japan) CT scanner. To obtain the perfusion images, a total of 19 acquisitions occurred over 60 s. All perfusion images were post processed using the commercial software MIStar (Apollo Medical Imaging Technology, Melbourne, Australia). Previously validated thresholds were applied in order to measure the volume of the acute perfusion lesion (relative delay time (DT) > 3 s) and acute ischemic core (relative cerebral blood flow – CBF- < 30% within the perfusion lesion) [20, 21]. Penumbral volume was calculated as the volume of the perfusion deficit (DT threshold > 3 s) minus the volume of the ischemic core (relative CBF threshold < 30% within the DT > 3 s lesion). The volume of DT > 6 and > 8 s lesions were also recorded when present. A mismatch ratio, a ratio between the extent of hypoperfused tissue at risk for infarction and the irreversibly damaged infarct core, was calculated by dividing DT3 lesion volume by the infarct core volume. Collateral status was assessed using the multiphase CTA reconstructed from CTP. Collaterals were graded according to the Alberta Stroke Program Early CT Score (ASPECTS) collateral score by a single neurologist [22, 23]. Follow-up DWI was performed (generally within 24 to 48 h) using Avanto 1.5 T or Vario 3 T scanners (Siemens, Munich, Germany).
WBV measurement
WBV was measured in duplicate within 2 h after sampling in accordance with the new guidelines for hemorheological laboratory techniques [24]. Five millilitres of EDTA blood sample was obtained from the patients prior to any stroke treatment. The measurement was carried out at 37°.C using a Brookfield DV-II + programmable viscometer (MA, USA), using a CP40 spindle. Whole blood adjusted to 40% hematocrit was used for the WBV measurement. Blood is a non-Newtonian fluid and its viscosity varies with the shear rate. For the present study, viscosity was measured at the shear rate of 20 s−1. This shear rate was chosen as it is the lowest possible shear rate that can provide accurate viscosity measures with the viscometer used. The low shear rate viscosity corresponds most closely with the environment in smaller blood vessels.
Viscometer performance was assessed against silicone viscosity standard fluid (Brookfield Amtek). Silicone Standards are Newtonian fluids which are accurate to ± 1% of viscosity value. The inter-assay co-efficient of variation obtained with this standard for WBV measurement was 8.2%.
Statistical analysis
Statistical analyses were performed with IBM SPSS Statistics 26 software. Descriptive results and quantitative baseline patient characteristics were presented as mean and standard deviation (SD) or median and interquartile range (IQR). Bivariate Pearson’s correlation was performed to examine the crude correlation of WBV with CTP parameters, DWI volume and stroke severity. For the parameters with significant correlation, a multivariate linear regression was used to confirm the associations after adjusting for the occlusion site and collateral status. Statistical significance was set at P < 0.05.
Results
Between September 2018 and February 2020, a total of 512 suspected stroke patients underwent acute CTP. Eighty-two confirmed ischemic stroke patients were recruited. Four hundred thirty patients were not included due to (a) after-hours visit (most of the cases); (b) unconfirmed ischemic stroke diagnosis; (c) not consented or (d) unable to obtain the blood sample before recanalization therapy.
Of the 82 recruited patients, the mean age was 72.7 (12.7) years and 47 (57%) were male. The median baseline NIHSS was 9 (4–17) (Table 1). Thirty-nine patients (47.5%) had a large vessel occlusion in baseline CTA. The most frequent stroke subtype was cardioembolic (n = 31, 38%) followed by cryptogenic (n = 26, 32%), large-artery atherosclerosis (n = 11, 14%), small-vessel occlusion (n = 10, 12%) and other determined causes (n = 3, 4%). In general, the WBV value was higher in cardioembolic and cryptogenic stroke compared to large-artery atherosclerosis and small-vessel occlusion, but the post hoc analysis did not reach the significant level (Table 2).
The median volume of hypoperfused lesion (DT3 lesion) and core was respectively 77.5 (40.2–140) and 13.5 (4.7–25.5) mL (Table 1). Half of the patients underwent follow-up MRI, with a median follow-up DWI volume of 11.0 (2–27) mL. Out of 82 patients, 7 patients were treated with alteplase alone (thrombolysis), 24 underwent endovascular thrombectomy alone and 6 patients received both therapies. WBV value is missing in 5 out of 82 patients, either due to insufficient blood volume or collection error. Collateral status assessment is missing in 1 out of 82 patients. This is due to lack of dynamic CTA as a result of imaging errors.
In the CTP images, DT3 lesion was seen in 63 patients, DT6 lesion was seen in 48 patients and DT8 lesion was seen in 37 patients. Similarly, 62 patients had ischaemic core lesion in CTP images.
WBV was significantly correlated with CTP parameters: perfusion lesion volume, ischemic core volume and mismatch ratio; DWI volume and baseline NIHSS (Table 3).
In multivariate linear regression models, WBV significantly predicted acute perfusion lesion volume (DT3), core volume and mismatch ratio (MMR) after adjusting for the occlusion site and collateral status (Table 4).
Discussion
The major finding of the present study was the significant association of WBV with baseline CTP parameters: DT3 lesion, MMR and core. The positive and significant association of WBV with baseline cerebral perfusion lesion (DT3) suggests that elevated WBV accentuates a reduction in blood flow in stroke. The association persists even after adjusting for the effect of collaterals and occlusion site. We observed the negative and significant association of WBV with mismatch ratio, after adjusting for the effect of collaterals and occlusion site. MMR is used in a clinical practice to select ischemic stroke patients for reperfusion therapies during the extended time window [25]. Decreased MMR and larger infarct size have also been shown to predict unfavourable stroke outcome [26]. A mild reduction in cerebral blood flow is normally corrected by local vasodilation (auto-regulation) to maintain cerebral perfusion [27]. Once the capacity of local vasodilation to compensate for a reduction in flow due to occlusion is overwhelmed (failed auto-regulation), the penumbra begins to infarct, after which this tissue is not salvageable despite restoration of cerebral perfusion. At the advent of a vessel occlusion (with corresponding reduction in cerebral blood flow and cerebral blood volume), residual flow via collateral vessels and the microcirculation determines the rate of infarct core growth. Elevated WBV will slow down microcirculation leading to an increased core, thereby decreasing the mismatch ratio, as seen in the present study.
Several previous studies have shown elevated WBV in stroke [6,7,8, 28,29,30,31,32,33,34]. However, whether the elevated WBV reduces cerebral reperfusion in stroke has not been definitely established. The present study demonstrated the independent association of WBV with MMR and perfusion and ischemic lesion volume. The present findings open new possibilities for therapeutic strategies targeting erythrocyte rheology to improve microcirculation. One such therapeutic agent, pentoxifylline, has been trialled in several small studies in the past. Though there are some positive reports on the benefit of this drug [35, 36], there is a lack of consistent evidence regarding its favourable effect on stroke outcome and these studies were conducted before the advent of modern recanalization therapy [37, 38]. Similarly, another potential therapeutic agent arginase inhibitor that improves blood rheology has shown some effect in increasing microvascular flow [39,40,41]. To verify the favourable outcome of such therapeutic agents that improves WBV and microcirculation, further rigorous clinical trials are warranted.
WBV value was found to be higher in cardioembolic stroke and cryptogenic stroke compared to large-artery atherosclerosis and small-vessel occlusion even though the difference did not reach the level of statistical significance in post hoc analysis. Moreover, there was no significant difference in the WBV between large-artery atherosclerosis and small-vessel occlusion. This is in contrast to the study of Song SH et al. [42], where the WBV in small-vessel occlusion was reported to be significantly higher than in large-artery atherosclerosis and other TOAST subtypes. This difference could be due to the shear rate chosen for the measurement of WBV. Song SH et al. measured WBV at the shear rate of 1 s−1. This reflects the shear rate in very small blood vessels (capillaries) aligning more with small-vessel occlusion. We measured WBV at the shear rate of 20 s−1. In the present study, WBV was significantly correlated with DWI lesion volume. However, after correcting for the effect of occlusion site, collaterals and recanalization status, the association was not significant. Nevertheless, we only had 41 patients with follow-up DWI lesion volume and 24 of them underwent recanalization therapy. Hence, larger sample size will be required to reach into any conclusion regarding the effect of WBV on infarct growth. Those who received follow-up MRI were more likely to have a lacunar stroke. We had 8 patients with lacunar stroke and all 8 had a follow-up MRI. In addition, patients who suffered very large strokes and were in palliative care after initial work-up typically did not receive follow-up MRI.
Our data showed lower median value of DWI lesion volume compared to CTP perfusion-derived core volume (Table 1). Median core volume analysed only among patient with follow-up DWI was also higher than DWI lesion. We measured core volume using a previously validated threshold in the acute perfusion lesion where acute ischemic core was defined as relative cerebral blood flow (CBF) < 30% compared to the contralateralhemisphere within the perfusion lesion [20, 21]. This threshold for defining core on CTP was established in patients receiving thrombolysis only and is probabilistic, measuring a pattern of blood flow which is incompatible with tissue survival in patients receiving thrombolysis alone. However, sometimes some of that tissue do actually survive, especially in patients who received ECR. In contrast, DWI actually measures dead tissue directly. Thus, Copen et al. suggested that CTP-derived CBV maps cannot reliably substitute for DWI in measuring core volume [43]. This explains why there are a few patients whose DWI lesion is smaller than their CTP core in our study.
The present study showed weak correlation of WBV with baseline NIHSS, but the significance was lost once the relationship was adjusted for occlusion site. No previous work on WBV has reported the association of WBV with stroke outcome measurements: NIHSS or modified Rankin Score. Hence, further study is warranted with larger sample size to consider the effect of WBV on infarct growth and clinical outcomes.
Limitation
We only had 41 patients with follow-up DWI. The sample size was too small to investigate the association of WBV with infarct growth.
Conclusion
We have demonstrated a significant correlation between WBV and baseline cerebral lesion volume. The use of CTP is continually growing in acute stroke diagnosis and management. Baseline CTP is routinely performed in stroke centres to increase diagnostic confidence by delineating the penumbra and ischemic core and identifying patients who may benefit from recanalization therapies [44]. Hence, association of WBV with clinically relevant imaging parameters of cerebral blood flow merits further investigation of role of WBV in cerebral haemodynamics of acute ischemic stroke. Lowering WBV pharmaceutically to increase cerebral microcirculation might be an adjunct therapy to boost cerebral reperfusion in acute ischemic stroke.
Availability of data and material
Data supporting the findings of this study are available from the corresponding author [PG] on request.
References
Cetin MS, Ozcan Cetin EH, Canpolat U, Aydın S, Temizhan A, Topaloglu S et al (2015) An overlooked parameter in coronary slow flow phenomenon: whole blood viscosity. Biomark Med 9(12):1311–1321
Cecchi E, Liotta AA, Gori AM, Valente S, Giglioli C, Lazzeri C et al (2009) Relationship between blood viscosity and infarct size in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Int J Cardiol 134(2):189–194
Santos-Galduróz RF, Bueno OFA, Yamaga LI, Armani F, Galduróz JCF (2012) Influence of blood viscosity to cerebral blood flow in older humans compared to young subjects. Clin Neurophysiol 123(1):117–120
Cetin MS, Ozcan Cetin EH, Balcı KG, Aydin S, Ediboglu E, Bayraktar MF et al (2016) The association between whole blood viscosity and coronary collateral circulation in patients with chronic total occlusion. Korean Circ J 46(6):784–790
TekinTak B, Ekizler FA, Cay S, Kafes H, Cetin EHO, Ozeke O et al (2020) Relationship between apical thrombus formation and blood viscosity in acute anterior myocardial infarction patients. Biomark Med 14(3):201–210
Lowe GDO, Lee AJ, Rumley A, Price JF, Fowkes FGR (1997) Blood viscosity and risk of cardiovascular events: the Edinburgh Artery Study. Br J Haematol 96(1):168–173
Velcheva I, Antonova N, Titianova E, Damianov P, Dimitrov N, Dimitrova V (2008) Hemorheological disturbances in cerebrovascular diseases. Clin Hemorheol Microcirc 39(1–4):391–396
Ernst E, Resch KL, Matrai A, Buhl M, Schlosser P, Paulsen HF (1991) Impaired blood rheology: a risk factor after stroke? J Intern Med 229(5):457–462
Fieschi C, Agnoli A, Battistini N, Bozzao Lt, Prencipe M (1968) Derangement of regional cerebral blood flow and of its regulatory mechanisms in acute cerebrovascular lesions. Neurology 18(12):1166
Wheeler HM, Mlynash M, Inoue M, Tipirnini A, Liggins J, Bammer R et al (2015) The growth rate of early DWI lesions is highly variable and associated with penumbral salvage and clinical outcomes following endovascular reperfusion. Int J Stroke 10(5):723–729
Hakimelahi R, Vachha BA, Copen WA, Papini GD, He J, Higazi MM et al (2014) Time and diffusion lesion size in major anterior circulation ischemic strokes. Stroke 45(10):2936–2941
Levy BI, Schiffrin EL, Mourad J-J, Agostini D, Vicaut E, Safar ME et al (2008) Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation 118(9):968–976
Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS (2011) Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol 10(10):909–921
Rafols JA (2015) Control of the brain microcirculation following traumatic brain injury and stroke. Brain Circ 1(2):146
Popel AS, Johnson PC (2005) Microcirculation and hemorheology. Annu Rev Fluid Mech 37:43–69
Walzl B, Walzl M, Valetitsch H, Lechner H (1995) Increased cerebral perfusion following reduction of fibrinogen and lipid fractions. Pathophysiol Haemost Thromb 25(3):137–143
Hartmann A (1985) Comparative randomized study of cerebral blood flow after long-term administration of pentoxifylline and co-dergocrine mesylate in patients with chronic cerebrovascular disease. Curr Med Res Opin 9(7):475–479
Hartmann A (1983) Effect of pentoxifylline on regional cerebral blood flow in patients with cerebral vascular disorders. Eur Neurol. 22((suppl 1)(Suppl. 1)):108–15
Grotta J, Ackerman R, Correia J, Fallick G, Chang J (1982) Whole blood viscosity parameters and cerebral blood flow. Stroke 13(3):296–301
Bivard A, Levi C, Krishnamurthy V, Hislop-Jambrich J, Salazar P, Jackson B et al (2014) Defining acute ischemic stroke tissue pathophysiology with whole brain CT perfusion. J Neuroradiol 41(5):307–315
Bivard A, Levi C, Spratt N, Parsons M (2013) Perfusion CT in acute stroke: a comprehensive analysis of infarct and penumbra. Radiology 267(2):543–550
Menon BK, d’Esterre CD, Qazi EM, Almekhlafi M, Hahn L, Demchuk AM et al (2015) Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology 275(2):510–520
Tian H, Chen C, Garcia-Esperon C, Parsons MW, Lin L, Levi CR et al (2019) Dynamic CT but not optimized multiphase CT angiography accurately identifies CT perfusion target mismatch ischemic stroke patients. Front Neurol 10:1130
Baskurt O, Boynard M, Cokelet G, Connes P, Cooke BM, Forconi S et al (2009) New guidelines for hemorheological laboratory techniques. Clin Hemorheol Microcirc. 42(2):75–97
Warach S (2001) New imaging strategies for patient selection for thrombolytic and neuroprotective therapies. Neurology 57(suppl 2):S48–S52
Gasparotti R, Grassi M, Mardighian D, Frigerio M, Pavia M, Liserre R et al (2009) Perfusion CT in patients with acute ischemic stroke treated with intra-arterial thrombolysis: predictive value of infarct core size on clinical outcome. Am J Neuroradiol 30(4):722–727
Heiss W (1983) Flow thresholds of functional and morphological damage of brain tissue. Stroke 14(3):329–331
Szapary L, Horvath B, Marton Z, Alexy T, Demeter N, Szots M et al (2004) Hemorheological disturbances in patients with chronic cerebrovascular diseases. Clin Hemorheol Microcirc 31(1):1–9
Kowal P, Marcinkowska-Gapińska A (2007) Hemorheological changes dependent on the time from the onset of ischemic stroke. J Neurol Sci 258(1):132–136
Tsuda Y, Satoh K, Kitadai M, Takahashi T (1997) Hemorheologic profiles of plasma fibrinogen and blood viscosity from silent to acute and chronic cerebral infarctions. J Neurol Sci 147(1):49–54
Fisher M, Meiselman HJ (1991) Hemorheological factors in cerebral ischemia. Stroke 22(9):1164–1169
Wong WJ, Hu HH, Luk YO, Lo YK (1994) The follow-up study of blood viscosity in the patients with acute ischemic stroke. Clin Hemorheol Microcirc 14(5):723–730
Furukawa K, Abumiya T, Sakai K, Hirano M, Osanai T, Shichinohe H et al (2016) Increased blood viscosity in ischemic stroke patients with small artery occlusion measured by an electromagnetic spinning sphere viscometer. J Stroke Cerebrovasc Dis 25(11):2762–2769
Totsimon K, Nagy A, Sandor B, Biro K, Csatho A, Szapary L et al (2016) Hemorheological alterations in carotid artery stenosis. Clin Hemorheol Microcirc 64(1):55–63
Chan Y, Kay C (1993) Pentoxifylline in the treatment of acute ischaemic stroke–a reappraisal in Chinese stroke patients. Clin Exp Neurol 30:110–116
Koppenhagen K, Wenig Hg, Müller K (1977) The effect of pentoxifylline (‘Trental’) on cerebral blood flow: a double-blind study. Curr Med Res Opin. 4(10):681–7
Hsu CY, Norris JW, Hogan EL, Bladin P, Dinsdale HB, Yatsu FM et al (1988) Pentoxifylline in acute nonhemorrhagic stroke. A randomized, placebo-controlled double-blind trial. Stroke. 19(6):716–22
Kruuse C, Jacobsen TB, Thomsen LL, Hasselbalch SG, Frandsen EK, Dige-Petersen H et al (2000) Effects of the non-selective phosphodiesterase inhibitor pentoxifylline on regional cerebral blood flow and large arteries in healthy subjects. Eur J Neurol 7(6):629–638
Mahdi A, Kövamees O, Checa A, Wheelock C, von Heijne M, Alvarsson M et al (2018) Arginase inhibition improves endothelial function in patients with type 2 diabetes mellitus despite intensive glucose-lowering therapy. J Intern Med 284(4):388–398
Shemyakin A, Kövamees O, Rafnsson A, Böhm F, Svenarud P, Settergren M et al (2012) Arginase inhibition improves endothelial function in patients with coronary artery disease and type 2 diabetes mellitus. Circulation 126(25):2943–2950
Pernow J, Mahdi A, Yang J, Zhou Z (2019) Red blood cell dysfunction: a new player in cardiovascular disease. Cardiovasc Res 115(11):1596–1605
Song SH, Kim JH, Lee JH, Yun YM, Choi DH, Kim HY (2017) Elevated blood viscosity is associated with cerebral small vessel disease in patients with acute ischemic stroke. BMC Neurol 17(1):1–10
Copen WA, Morais LT, Wu O, Schwamm LH, Schaefer PW, González RG et al (2015) In acute stroke, can CT perfusion-derived cerebral blood volume maps substitute for diffusion-weighted imaging in identifying the ischemic core? PLoS One. 10(7):e0133566
Bivard A, Levi C, Krishnamurthy V, McElduff P, Miteff F, Spratt NJ et al (2015) Perfusion computed tomography to assist decision making for stroke thrombolysis. Brain 138(7):1919–1931
Funding
This work was supported by the Hunter Medical Research Institute (grant number G1801359); Priority Research Centre for Stroke and Brain Injury, The University of Newcastle and National Health and Medical Research Council Project Grant (APPID 1085550).
Author information
Authors and Affiliations
Contributions
PG and TL worked on ethics application (variation), proposal development, patient recruitment, WBV measurement, manuscript drafting; ST performed collateral assessment; AB performed CT perfusion image analysis, imaging data interpretation; EH contributed to the overall manuscript editing and logistics for patient recruitment, provided suggestion for data analysis and data management; MP and CL provided comments and suggestions for critical manuscript revision, contributed valuable discussions; CGE and NS coordinated patient recruitment in the hospital, obtained clinical data, edited manuscript, participated in study design and manuscript revision.
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the Hunter New England Health District human research ethics committee (14/10/15/4.02). Patients (or their guardians) have given their written informed consent.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gyawali, P., Lillicrap, T.P., Tomari, S. et al. Whole blood viscosity is associated with baseline cerebral perfusion in acute ischemic stroke. Neurol Sci 43, 2375–2381 (2022). https://doi.org/10.1007/s10072-021-05666-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05666-5