Abstract
Animal vocalisations encode a wide range of biological information about the age, sex, body size, and social status of the emitter. Moreover, vocalisations play a significant role in signalling the identity of the emitter to conspecifics. Recent studies have shown that, in the African penguin (Spheniscus demersus), acoustic cues to individual identity are encoded in the fundamental frequency (F0) and resonance frequencies (formants) of the vocal tract. However, although penguins are known to produce vocalisations where F0 and formants vary among individuals, it remains to be tested whether the receivers can perceive and use such information in the individual recognition process. In this study, using the Habituation-Dishabituation (HD) paradigm, we tested the hypothesis that penguins perceive and respond to a shift of ± 20% (corresponding to the natural inter-individual variation observed in ex-situ colonies) of F0 and formant dispersion (ΔF) of species-specific calls. We found that penguins were more likely to look rapidly and for longer at the source of the sound when F0 and formants of the calls were manipulated, indicating that they could perceive variations of these parameters in the vocal signals. Our findings provide the first experimental evidence that, in the African penguin, listeners can perceive changes in F0 and formants, which can be used by the receiver as potential cues for the individual discrimination of the emitter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A crucial aspect in the study of animal vocal communication is the identification of the acoustic parameters that may encode salient information for the receiver (Owings and Morton 1998; Seyfarth and Cheney 2010). The application of the source-filter theory for human voice production (Fant 1980) to nonhuman animals’ vocalisations (Beckers et al. 2004; Taylor and Reby 2010; Budka and Osiejuk 2013; Vannoni and McElligott 2008) and the use of vocal tract modelling approaches (Gamba et al. 2017; Reby et al. 2018) have advanced our knowledge of the use of source-and filter-related vocal parameters to convey indexical and individual identity information in bird and mammal vocal signals. In penguins, the source-filter theory approach allowed a better understanding of the vocal repertoire of the African penguin (Spheniscus demersus) and the role of the independent contribution of the different organs of the respiratory system on vocal production (lung—duration and temporal patterns; syrinx—source, determining the fundamental frequency; the vocal tract—filter, generating formant frequencies) (Favaro et al. 2015). Moreover, recent studies have demonstrated that in the African penguin, at least two vocal types, namely the contact calls and the Ecstatic Display Songs (EDS), encode information on the individual identity of the emitter (Favaro et al. 2016, 2017). In these vocal types, the fundamental frequency (F0) and the formants are essential cues to assign the identity of the callers. In addition, based on the relative stability of contact calls and EDS, it is possible to identify individuals reliably over consecutive breeding seasons (Calcari et al. 2021).
Considering the relative importance that vocalisations play in penguins’ social life, such as group cohesion, mitigation of conflicts and recognition between mates, parents, and offspring, it is reasonable to expect that penguins can attend to temporal (duration, rhythm), source-related (F0), and filter-related (formants) parameters encoded in their calls (Lengagne et al. 1997; Aubin and Jouventin 2002a; Jouventin and Aubin 2002; Favaro et al. 2015; Jouventin and Dobson 2018). Indeed, previous studies have shown that non-nesting penguins of the genus Aptenodytes attend to the beats generated by the interaction of the two fundamental frequencies produced in their syrinx (i.e., two-voice system) to infer the individual identity of conspecifics (Aubin et al. 2000), while, nesting species, such as the Adélie (Pygoscelis adeliae) or the Gentoo (P. papua) penguins, pay more attention to the spectral profile and the precise frequency values of the harmonics (Aubin and Jouventin 2002b). These differences in attending to F0 and formants of the vocalisations have evolved in response to different breeding systems (non-nesting vs nesting), socio-ecological pressure, and the level of recognition needs across the different species (Jouventin and Aubin 2002). Even though it has been shown that source-and filter-related parameters encode meaningful biological information in penguin species, the investigation of the perceptual and functional relevance of these vocal parameters from the listeners’ perspective is lacking and unexplored in the African penguin.
The investigation of the receiver's ability to recognize an individual as unique can reveal important aspects of communicative abilities on colonial life. Here, by using re-synthesised contact calls of different African penguins in combination with the habituation-dishabituation paradigm, we tested the hypothesis that African penguins perceive and respond to a shift in the F0 and formant dispersion (ΔF) of their vocalisations within the species-specific variability of these parameters. The HD paradigm is a powerful behavioural paradigm investigating perceptual abilities in nonhuman animals (Rendall et al. 1996; Charlton et al. 2011a; Baciadonna et al. 2019; Carlson et al. 2020). This paradigm estimates the ability to discriminate whether two stimuli are perceived differently based on the behavioural responses they elicit. When a stimulus is presented continuously, the subject’s attention towards it declines (habituate). Instead, when a new stimulus is presented, the subject's attention is renewed if the stimulus is perceived as different from the previous one (dishabituate). In detail, we predicted that after a reduced response to conspecific contact calls, penguins would show a renewed response when the fundamental frequency or formant dispersion is increased or decreased by 20% from their original frequency values. If confirmed, our study will enhance our understanding on the penguins’ perception of the information encoded in the spectral envelope of their species-specific vocalisations, demonstrating that source- and filter-related acoustic parameters likely play a crucial role for individual recognition.
Methods
Subjects
This study was conducted at Zoom Torino (Italy) between February 2021 and April 2022. At the beginning of the study, the colony consisted of 37 adult penguins born in four different zoological facilities in Europe (Artis Royal Zoo, Amsterdam, NL; Bird Park Avifauna, Alphen an den Rijn, NL; Wilhelma Zoo, Stuttgart, DE; South Lake Wild Animal Park, Manchester, UK). However, during the study period, the number of penguins in the colony changed. In March 2021, 23 penguins were relocated, while between August and October 2021 a group of 16 penguins was added to the colony (from Zoo Wrocław, Wrocław, PL; Safari de Peaugres, Lyon, FR) bringing the total number to 30 penguins. Overall, a total of 14 adult African penguins (eight males and seven females, age range: 1–34 y.o.) were tested. Two penguins passed away during data collection and could not be tested in all experimental conditions.
Acoustic stimuli
We selected the acoustic stimuli from a database of 120 contact calls belonging to 20 individuals (10 males and ten females) from two non-familiar penguin colonies housed at Zoomarine (Pomezia, Italy) and Zoological Garden of Pistoia (Pistoia, Italy). We collected the acoustic data from the Zoomarine colony between February and October 2020 (7.00–13.00; 44 days; 220 h total). We recorded at the Zoological Garden of Pistoia between October 2016 and June 2017 (8.00–18.00; 68 days; 230 h total). We collected all recordings between 3 to 10 m from the vocalizing individuals with a RODE NTG-2 shotgun microphone (flat frequency response 20 Hz to 20 kHz, max SPL 131 dB) connected to a ZOOM H5 handy recorder (48 kHz sampling rate). We saved audio files in WAV format (16-bit amplitude resolution) and stored them on a secure digital memory card.
Playback sequences and procedures
We used the HD paradigm to investigate whether penguins could perceive and discriminate conspecific contact calls when the F0 and ΔF were modified one at a time (Carlson et al. 2020). This paradigm habituates an individual to a stimulus through repeated stimulus presentation (reduction of responses). The presentation of a dishabituation stimulus follows habituation. If the individual pays attention to the new stimulus, this would indicate an ability to perceive and discriminate the change between the two stimuli. Furthermore, this paradigm provides a control condition (i.e., rehabituation) to exclude the possibility that the dishabituation reaction was not simply due to a spontaneous recovery of the pre-habituation level. This control is achieved by presenting a stimulus previously encountered during the habituation phase after the dishabituation phase (Charlton et al. 2011a; Baciadonna et al. 2019).
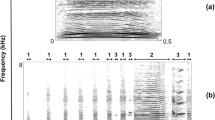
For each non-familiar donor individual (N = 20), we selected six good-quality contact calls with low background noise (a total of 120 contact calls) to build 40 playback sequences. Each sequence comprised seven contact calls separated by a time interval of 15 s. Four calls from the donor sequence were selected, concatenated randomly, and used to build the habituation phase (H1–H4) and build two playback sequences. We used each donor's other two contact calls as the unique and last contact call of the habituation phase (H5), which we concatenated to the contact calls H1–H4 of the sequences. The dishabituation phase (D, call 6) was created by modifying the last call of habituation (H5). Although call D was constructed using H5, the manipulation of the acoustic parameters led to an entirely unrelated novel acoustic stimulus (Fig. 1). It is still possible that the penguin does not perceive the shift in acoustic parameters, and thus, in line with the assumptions of the HD paradigm, repeated exposure to the same stimulus will lead to the habituation. To shift the fundamental frequencies, we calculated, using Praat v. 6.1.5 (Boersma and Weenink 2022), the fundamental frequency (F0 mean) of the H5. Subsequently, the F0 mean of H5 was increased or decreased by 20% by using the Convert > > Change gender function > > New pitch median (Hz) tab in Praat and saved as a new mono file in wav format (16-bit resolution). We chose the 20% variation in the fundamental frequency and formants considering the inter-individual variation of F0 and ΔF observed in the contact calls of ex-situ African penguin colonies (Favaro et al. 2015).
Sequence of contact calls used in a playback experiment, shift F0 + 20%. H Habituation phase, D Dishabituation phase, R Rehabituation phase. The arrow indicates the call with F0 increased by 20%. Figure created with the R package ‘seewave’ v. 2.2.0 (Sueur et al. 2008). Spectrogram parameters: window length = 10,000, overlap = 60, window type = ‘Hann’
We used the formant shift ratio tab on Praat to increase or decrease the ΔF by inserting 1.2 or 0.8 values. Finally, for the rehabituation phase (R, calls 7), we concatenated the last call used in the habituation phase (H5) to the playback sequences. The original duration of the calls was left unchanged, and we equalised the peak amplitude of calls during the preparation of playback sequences. We broadcast playback sequences from a Bose® Soundlink Mini II loudspeaker connected wirelessly to an Oppo® A72 smartphone at an approximately natural amplitude (72.40 ± 2.47 dB) measured at 1 m using a Monacor® SM-2 sound level meter.
We presented four different playback sequences for each tested penguin: two for each acoustic parameter (F0 and ΔF) shifted + 20% and − 20%. Before the experiment started, the experimenter inspected each nest available in the colony. When we found a penguin in the nest, the experimenter identified the subjects by using a coloured flipper band located on the wings. After the identification, the experimenter placed a video camera Sony® (HDR-CX140) and the speaker (aligned) 5 m away from the nest and moved away from the penguin’s view. After approx. 3–5 min, the experimenter played the acoustic sequence selected for the subjects remotely. At the end of the playback sequences, the experimenter approached the nest to remove the camera and the speaker. Each subject was never tested more than twice on the same day, and we allocated a minimum of 1 h break between each playback presentation.
Scoring of the behavioural responses
The duration of the first looking (s) at the speaker and latency (s) were measured. We defined latency as the amount of time that elapses between the onset of the stimulus and any head movement towards the speaker. We assigned a maximum time of 15 s for the latency for subjects that did not respond. We defined the duration of first looking as the time the penguin looked at the speaker from the end of the latency until any head movement occurred. Without latency, this behaviour was not scored, including when the penguin was already directed toward the speaker. Subjects’ responses for each call included in each playback sequence were analysed using BORIS v. 7.10.7 (Friard and Gamba 2016). We tested the reliability of the parameters measured, scoring 20% of the sessions to test them by the two observers. The interclass correlation coefficient calculated for all the behaviours analysed statistically was: 0.84 for the duration of first looking and 0.84 for latency.
Statistical analyses
We used the software R version 4.1.0 (R Development Core Team 2021) for statistical analyses. The first step was to establish whether penguins habituated to the sounds by comparing the duration of the first look and latency of the five calls (H1–H5) played during the habituation phase.
Subsequently, in the discrimination phase, the last habituation call (H5) was compared with the dishabituation call (D), and the duration of the first look to the dishabituation call was compared to the rehabituation call (R) to establish whether penguins were able to detect any differences between these calls.
The duration of the first look was analysed using a Generalised Linear Mixed Model (GLMM) using the lme4 package (Bates et al. 2015). The model included the duration of the first look (log-transformed) as the response variable, Phase (H1 to H5 for the habituation phase and H5, D and R for the discrimination phase), Condition (Fundamental frequency and Formants), and Shift frequencies (− 20% and + 20%) as fixed factors. We assessed the significance of the full model by comparing this model with the model that included only the random factors (null model) using a likelihood ratio test. We checked the model fit and over‐dispersion using the DHARMa 0.3.3.0 package (Hartig 2020). The p-value of each factor was derived using the “drop1” function (Barr et al. 2013). Also, the subjects’ identity was included as a random factor to control for repeated measurements of the same subject in all models performed. Finally, we performed pairwise comparisons using the lsmeans multiple contrast package (Lenth 2016) with a Tukey post hoc test.
We analysed latency with Cox proportional hazards models with the function coxme in the R package Survival. The model included latency as the response variable, Phase (H1 to H5 for the habituation phase and H5, D and R for the discrimination phase), Condition (Fundamental frequency and Formants), and Shift frequencies (− 20% and + 20%) as fixed factors. Also, the subjects’ identity was included as a random factor to control for repeated measurements of the same subject in all models performed. The p-value of each factor was derived using the ANOVA function followed by a Tukey post hoc test to account for pairwise comparisons. We deemed subjects that did not respond with 15 s as censored results.
Results
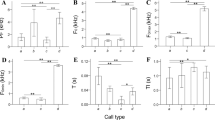
During the habituation phase, the duration of looking at the playback calls gradually decreased (Table 1). Post-hoc analyses revealed that the duration of the first look at call H1 (mean ± SE = 6.27 ± 0.61 s) was significantly longer compared to the duration of the first look at call H5 (mean ± SE = 3.33 ± 0.44 s; estimate = − 0.60, s.e. = 0.12, z = − 4.74, p < 0.001). Neither condition nor shifted F0 and ΔF predicted the duration of the first look during habituation (Table 1). The duration of the first look was predicted by phase (Table 1) when H5, D and R were considered (Table 1, Fig. 2A). Penguins significantly increased the duration of looking between the dishabituation playback (D; mean ± SE = 5.43 ± 0.48 s) and the last playback of the habituation phase (H5; estimate = 0.45, s.e. = 0.13, z = 3.26, p = 0.003; Fig. 2A). In addition, penguins significantly reduced the duration of the first look between the rehabituation playback (R; mean ± SE = 3.65 ± 0.44 s), and the dishabituation call (D; estimate = − 0.35, s.e. = 0.13, z = − 2.55, p = 0.028; Fig. 2A), with a similar first-look duration of the last habituation playback (H5). Neither condition nor shifted F0 and ΔF predicted the duration of the first look during habituation (Table 1).
Behavioural response of penguins during playback experiments. A Duration of the first look to the habituation–dishabituation playback sequences (N = 14). The box plots presented here (which illustrate horizontal lines = median; black squares = mean; boxes extend from lower to upper quartile, and whiskers indicate interquartile range above the upper quartile (max) or below the lower quartile (min), show an initial diminution of response levels across the habituation phase (H1–H5) followed by a renewal of response levels to the dishabituation stimulus (D). Finally, a decrease in response levels after the rehabituation stimulus, returning to that of the last playback of the habituation phase (H5); B probability during the habituation phase of penguins to respond at the playback calls; C probability during the H5, D and R calls of penguins to respond at the playback
During the habituation phase, latency to look at the playback calls gradually increased (Table 2, Fig. 2B). Post-hoc analyses revealed that the latency at call H1 (mean ± SE = 2.12 ± 0.29 s) was significantly shorter compared to the latency at call H5 (mean ± SE = 5.17 ± 0.72 s; estimate = − 0.87, s.e. = 0.21, z = − 4.12, p < 0.001). Neither condition nor shifted F0 and ΔF predicted latency during habituation (Table 2, Fig. 2A). The latency to look at playback calls was predicted by phase (Table 2) when H5, D and R were considered (Table 2, Fig. 2C).
Penguins looked faster between the dishabituation playback (D; mean ± SE = 2.96 ± 0.55 s) and the last playback of the habituation phase (H5; estimate = 0.75, s.e. = 0.21, z = 3.46, p = 0.0015; Fig. 2C). The latency to look at the playback calls between the rehabituation playback (R; mean ± SE = 3.95 ± 0.62 s) and the dishabituation call was longer, despite the result being not significant (D; estimate = − 0.47, s.e. = 0.21, z = − 2.18, p = 0.073, Fig. 2C). Neither condition nor shifted F0 and ΔF predicted the duration of the first look during habituation (Table 2).
Discussion
The study provides convincing evidence that African penguins attend to F0 and ΔF of their species-specific vocalisations. Penguins showed a significant renewal of response (i.e., duration of first looking) when hearing the contact calls in which F0 or the ΔF had been shifted from their initial frequencies during the habituation phase. The significantly reduced response to the rehabituation phase indicates that the response to the shifted contact calls was not simply a recovery of the pre-habituation response level. Therefore, our findings demonstrate that penguins perceive and respond to changes in fundamental frequency and formant dispersion within the natural range of variation and that these differences in shift (± 20%) were enough to be detected. Furthermore, the spontaneous penguins’ responses (i.e., in the absence of training) suggest the functional significance of F0 and formants for the vocal communication system in the African penguin.
Although contact calls are one of the most common type of calls used in a variety of animal species and most likely have evolved primarily to maintain group cohesion (Kondo and Watanabe 2009), a growing body of evidence has suggested that contact calls can be used in individual recognition, especially in fission–fusion social systems in which small group of individuals disperse during the foraging activity and later aggregate in larger groups (Macedonia 1986; Mathevon 1997; Wanker and Fischer 2001; Sharp and Hatchwell 2005; Buhrman-Deever et al. 2008, Mumm et al. 2014). Similarly, in the African penguin, contact calls are emitted to maintain group cohesion when visually isolated from other individuals (Favaro et al. 2015, 2016), often when foraging at sea (McInnes et al. 2020). Furthermore, in ex-situ colonies, it is common to observe adult penguins emitting contact calls to keep contact specifically with their partner (Baciadonna et al. 2021, 2022) or juveniles uttering these vocalisations in the presence of the keepers (F.T. personal observation). Beyond the African penguins, recent findings have provided convincing evidence that the fundamental frequency of the contact calls play a role also for colony-mate recognition in different animal species: birds (Forpus passerines; Berg et al. 2011), cervids (adult female of fallow deer; Torriani et al. 2006), bats (Antrozous pallidus; Arnold and Wilkinson 2011).
The ability of penguins to detect slight variations in the F0 and formants of manipulated contact calls further emphasizes the significance of these acoustic parameters in conveying biologically meaningful information across a wide range of animal taxa, even those that are phylogenetically distant (Taylor & Reby 2010). Previous studies demonstrated that humans are sensitive to frequency spacing shifts of about 4% in speech (Smith et al. 2005; Puts et al. 2007; Monahan and Idsardi 2010), while non-human mammals like the red deer (Charlton et al. 2007a) and the giant panda (Charlton et al. 2010) can perceive shifts of 5–10% in their species-specific calls. Our results showed that the African penguins can detect a shift in F0 and formant spacing of 20%. However, a possible limitation of this study is the lack of determining a precise threshold sensitivity of African penguins to the variation of these vocal parameters. Further studies are needed to investigate these aspects in detail.
The duration of the first look toward the speaker suggests that penguins were equally attentive regardless of conditions (F0 or ΔF) and the type of manipulation applied (± 20%) to the dishabituation stimulus. Penguins’ latency across the five calls increased as expected regardless of the conditions and the type of re-synthesised signal considered indicative that they gradually habituated. In addition, penguins responded significantly faster to the dishabituation call regardless of the conditions and the shift we applied. By contrast, penguins’ response to the rehabituation call was not different from the last call of habituation, although the mean latency decreased. According to the habituation-dishabituation paradigm, this response indicates that these two calls were different enough to be perceived by the penguins. Conforming to the methodological paradigm to validate the robustness of behavioural responses that occurred during the presentation of the dishabituation calls, we should expect a similar behavioural response (i.e., mean latency) observed in the last call of habituation. According to our prediction, the unexpected behavioural pattern observed would suggest that the response to the dishabituation calls was simply a spontaneous rebound of pre-habituation response levels. Although this is plausible, we cannot exclude other possible explanations, especially because penguins' latency to react between the rehabituation playback and the dishabituation call was longer, despite the results not being significant. It is also possible that latency is more indicative of general penguins' attention/alertness towards external stimuli, and if relevant, they might allocate more time, as we had observed when the duration of the first look was included. Further research is necessary to interpret this result in a broader perspective. For instance, measuring physiological parameters, such as the heart rate and the heart rate variability, could provide additional information about the responses of the penguins’ sympathetic and parasympathetic system during the playback of the dishabituation stimulus (Baciadonna et al. 2019).
Spontaneous perception of F0 or ΔF in species-specific vocalisations has been demonstrated in several mammals (Charlton 2007a; Charlton et al. 2008a, b; Charlton et al. 2010, 2011a; Ghazanfar et al. 2007; Reby et al. 2005; Fitch and Fritz 2006; Searby and Jouventin 2003) and a few bird species (Fitch and Kelley 2000; Vignal et al. 2004; for a review: Dooling et al. 2000), and quite often the targeting aspect investigated is the perception of formant shift. The emphasis on filter-related acoustic parameters in mammals is due to different reasons: first, they encode cues to individual identity (Rendall et al. 1998; Fitch 1997; McComb et al. 2003; Vannoni and McElligott 2007; Charlton 2011b; Green et al. 2019); second because they are honest cues to body size compared to the source-related parameters with implications in mate selection (Fitch 1997, 2000; Charlton et al. 2007b; Reby and McComb 2003; Vannoni and McElligott 2008; Taylor et al. 2010) and, finally because the formants play a role in vowel perception in human speech (Hillenbrand and Clark 2009; Root-Gutteridge et al. 2019). Investigating the perceptual and functional role of vocalisations in other species can help us to reconstruct the evolution of complex vocal communication system (Garcia and Favaro 2017; Fitch 2010).
Here, we provide evidence that penguins discriminate source- and filter-related components of contact calls which have been indicated in previous studies as the primary cues to individual identity in penguins of the genus Spheniscus (Favaro et al. 2015; 2016; 2017; Calcari et al. 2021). Further studies using a similar methodological paradigm are needed to investigate whether penguins can discriminate changes in other acoustic parameters potentially relevant to their body size and mass. These might include the duration of the vocal units and the composition and temporal patterns of the vocal sequences (Favaro et al. 2020). Playback experiments, especially in wild colonies, could also be relevant to determine how subjects use the source- and filter-related acoustic information in mate choice and intersexual competitions. In summary, our findings on penguins’ responses to variation in fundamental frequency and formants dispersion of species-specific calls suggest that these aspects play an essential evolutionary functional role in the animal communication system and pave the way for further comparative studies.
Data availability
Datasets generated and/or analysed during the current study are available from the corresponding author upon request.
References
Arnold BD, Wilkinson GS (2011) Individual specific contact calls of pallid bats (Antrozous pallidus) attract conspecifics at roosting sites. Behav Ecol Sociobiol 65:1581–1593. https://doi.org/10.1007/s00265-011-1168-4
ASAB Ethical Committee, and ABS Animal Care Committee (2022) Guidelines for the treatment of animals in behavioural research and teaching. Anim Behav 183:I–XI. https://doi.org/10.1016/s0003-3472(21)00389-4
Aubin T, Jouventin P (2002a) Localisation of an acoustic signal in a noisy environment: the display call of the king penguin Aptenodytes patagonicus. J Exp Biol 205:3793–3798. https://doi.org/10.1242/jeb.205.24.3793
Aubin T, Jouventin P (2002b) How to vocally identify kin in a crowd: the penguin model. Adv Study Behav 31:243–277. https://doi.org/10.1016/s0065-3454(02)80010-9
Aubin T, Jouventin P, Hildebrand C (2000) Penguins use the two–voice system to recognize each other. Proc R Soc B 267:1081–1087. https://doi.org/10.1098/rspb.2000.1112
Baciadonna L, Briefer EF, Favaro L, McElligott AG (2019) Goats distinguish between positive and negative emotion-linked vocalisations. Front Zool 16:25. https://doi.org/10.1186/s12983-019-0323-z
Baciadonna L, Solvi C, La Cava S, Pilenga C, Gamba M, Favaro L (2021) Cross-modal individual recognition in the African penguin and the effect of partnership. Proc R Soc B 288:20211463. https://doi.org/10.1098/rspb.2021.1463
Baciadonna L, Solvi C, del Vecchio F, Pilenga C, Baracchi D, Bandoli F, Isaja V, Gamba M, Favaro L (2022) Vocal accommodation in penguins (Spheniscus demersus) as a result of social environment. Proc R Soc B 289:20220626. https://doi.org/10.1098/rspb.2022.0626
Barr DJ, Levy R, Scheepers C, Tily HJ (2013) Random effects structure for confirmatory hypothesis testing: Keep it maximal. J Mem Lang 68:255–278. https://doi.org/10.1016/j.jml2012.11.001
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Beckers GJL, Nelson BS, Suthers RA (2004) Vocal-Tract filtering by lingual articulation in a parrot. Curr Biol 14:1592–1597. https://doi.org/10.1016/j.cub.2004.08.057
Berg KS, Delgado S, Okawa R, Beissinger SR, Bradbury JW (2011) Contact calls are used for individual mate recognition in free-ranging green-rumped parrotlets, Forpus passerinus. Anim Behav 81:241–248. https://doi.org/10.1016/j.anbehav.2010.10.012
Boersma P, Weenink D (2022) Praat: doing phonetics by computer [Computer program]. Version 6.3.03. http://www.praat.org/
Budka M, Osiejuk TS (2013) Formant frequencies are acoustic cues to caller discrimination and are a weak indicator of the body size of corncrake males. Ethology 119(11):960–969. https://doi.org/10.1111/eth.12141
Buhrman-Deever SC, Hobson EA, Hobson AD (2008) Individual recognition and selective responses to contact calls in foraging brown-throated conures, Aratinga pertinax. Anim Behav 76:1715–1725. https://doi.org/10.1016/j.anbehav.2008.08.007
Calcari C, Pilenga C, Baciadonna L, Gamba M, Favaro L (2021) Long-term stability of vocal individuality cues in a territorial and monogamous seabird. Anim Cogn 24:1165–1169. https://doi.org/10.1007/s10071-021-01518-z
Carlson NV, Kelly EMK, Couzin I (2020) Individual vocal recognition across taxa: a review of the literature and a look into the future. Phil Trans R Soc B 375:20190479. https://doi.org/10.1098/rstb.2019.0479
Charlton BD, Reby D, McComb K (2007a) Female perception of size-related formant shifts in red deer, Cervus elaphus. Anim Behav 74:707–714. https://doi.org/10.1016/j.anbehav.2006.09.021
Charlton BD, Reby D, McComb K (2007b) Female red deer prefer the roars of larger males. Biol Lett 3:382–385. https://doi.org/10.1098/rsbl.2007.0244
Charlton BD, McComb K, Reby D (2008a) Free-ranging red deer hinds show greater attentiveness to roars with formant frequencies typical of young males. Ethology 114:1023–1031
Charlton BD, Reby D, McComb K (2008b) Effect of combined source (F0) and filter (formant) variation on red deer hind responses to male roars. J Acoust Soc Am 123:2936–2943. https://doi.org/10.1121/1.2896758
Charlton BD, Zhihe Z, Snyder R (2010) Giant pandas perceive and attend to formant frequency variation in male bleats. Anim Behav 79:1221–1227. https://doi.org/10.1016/j.anbehav.2010.02.018
Charlton BD, Ellis WAH, McKinnon AJ, Brumm J, Nilsson K, Fitch WT (2011a) Perception of male caller identity in koalas (Phascolarctos cinereus): acoustic analysis and playback experiments. PLoS ONE 6:e20329. https://doi.org/10.1371/journal.pone.0020329
Charlton BD, Ellis WAH, McKinnon AJ, Cowin GJ, Brumm J, Nilsson K, Fitch WT (2011b) Cues to body size in the formant spacing of male koala (Phascolarctos cinereus) bellows: honesty in an exaggerated trait. J Exp Biol 214:3414–3422. https://doi.org/10.1242/jeb.061358
Dooling RJ, Lohr B, Dent ML (2000) Hearing in birds and reptiles. Springer, New York, pp 308–359. https://doi.org/10.1007/978-1-4612-1182-2_7
Fant G (1980) The relations between area functions and the acoustic signal. Phonetica 37:55–86. https://doi.org/10.1159/000259983
Favaro L, Gamba M, Alfieri C, Pessani D, McElligott AG (2015) Vocal individuality cues in the African penguin (Spheniscus demersus): a source-filter theory approach. Sci Rep 5:17255. https://doi.org/10.1038/srep17255
Favaro L, Gili C, Da Rugna C, Gnone G, Fissore C, Sanchez D, McElligott AG, Gamba M, Pessani D (2016) Vocal individuality and species divergence in the contact calls of banded penguins. Behav Processes 128:83–88. https://doi.org/10.1016/j.beproc.2016.04.010
Favaro L, Gamba M, Gili C, Pessani D (2017) Acoustic correlates of body size and individual identity in banded penguins. PLoS ONE 12:e0170001. https://doi.org/10.1371/journal.pone.0170001
Favaro L, Gamba M, Cresta E, Fumagalli E, Bandoli F, Pilenga C, Isaja V, Mathevon N, Reby D (2020) Do penguins’ vocal sequences conform to linguistic laws? Biol Lett 16:20190589. https://doi.org/10.1098/rsbl.2019.0589
Fitch WT (1997) Vocal tract length and formant frequency dispersion correlate with body size in rhesus macaques. J Acoust Soc Am 101:3136–3136. https://doi.org/10.1121/1.419022
Fitch WT (2000) Skull dimensions in relation to body size in nonhuman mammals: the causal bases for acoustic allometry. Zoology 103:40–58
Fitch WT (2010) The evolution of language. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9780511817779
Fitch WT, Fritz JB (2006) Rhesus macaques spontaneously perceive formants in conspecific vocalisations. J Acoust Soc Am 120:2132–2141
Fitch WT, Kelley JP (2000) Perception of vocal tract resonances by whooping cranes Grus americana. Ethology 106:559–574. https://doi.org/10.1046/j.1439-0310.2000.00572.x
Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol 7:1325–1330. https://doi.org/10.1111/2041-210X.12584
Gamba M, Favaro L, Araldi A, Matteucci V, Giacoma C, Friard O (2017) Modeling individual vocal differences in group-living lemurs using vocal tract morphology. Curr Zool 63:467–475. https://doi.org/10.1093/cz/zox023
Garcia M, Favaro L (2017) Animal vocal communication: function, structures, and production mechanisms. Curr Zool 63:417–419. https://doi.org/10.1093/cz/zox040
Ghazanfar A, Turesson H, Maier J, Vandinther R, Patterson RD, Logothetis N (2007) Vocal-tract resonances as indexical cues in rhesus monkeys. Curr Biol 17:425–430. https://doi.org/10.1016/j.cub.2007.01.029
Green A, Clark C, Favaro L, Lomax S, Reby D (2019) Vocal individuality of Holstein-Friesian cattle is maintained across putatively positive and negative farming contexts. Sci Rep 9:18468. https://doi.org/10.1038/s41598-019-54968-4
Hartig F (2020) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. https://CRAN.R-project.org/package=DHARMa.
Hillenbrand JM, Clark MJ (2009) The role of F0 and formant frequencies in distinguishing the voices of men and women. Atten Percept Psychophys 71:1150–1166. https://doi.org/10.3758/app.71.5.1150
Jouventin P, Aubin T (2002) Acoustic systems are adapted to breeding ecologies: individual recognition in nesting penguins. Anim Behav 64:747–757. https://doi.org/10.1006/anbe.2002.4002
Jouventin P, Dobson SF (2018) Why penguins communicate: the evolution of visual and vocal signals. Elsevier Academic Press, Amsterdam. https://doi.org/10.1016/c2016-0-01088-4
Kondo N, Watanabe S (2009) Contact calls: information and social function. Jpn Psychol Res 51:197–208. https://doi.org/10.1111/j.1468-5884.2009.00399.x
Lengagne T, Lauga J, Jouventin P (1997) A method of independent time and frequency decomposition of bioacoustic signals: inter-individual recognition in four species of penguins. Comptes Rendus De L’académie Des Sciences-Series III-Sciences De La Vie 320(11):885–891. https://doi.org/10.1016/s0764-4469(97)80873-6
Lenth RV (2016) Least-squares means: the R package lsmeans. J Stat Softw 69:1–33. https://doi.org/10.18637/jss.v069.i01
Macedonia JM (1986) Individuality in a contact call of the ringtailed lemur (Lemur catta). Am J Primatol 11:163–179. https://doi.org/10.1002/ajp.1350110208
Mathevon N (1997) Individuality of contact calls in the Greater Flamingo Phoenicopterus ruber and the problem of background noise in a colony. Ibis 139:513–517. https://doi.org/10.1111/j.1474-919x.1997.tb04667.x
McComb K, Reby D, Baker L, Moss C, Sayialel S (2003) Long-distance communication of acoustic cues to social identity in African elephants. Anim Behav 65:317–329. https://doi.org/10.1006/anbe.2003.2047
McInnes AM, Thiebault A, Cloete T, Pichegru L, Aubin T, McGeorge C, Pistorius PA (2020) Social context and prey composition are associated with calling behaviour in a diving seabird. Ibis 162:1047–1059. https://doi.org/10.1111/ibi.12806
Monahan PJ, Idsardi WJ (2010) Auditory sensitivity to formant ratios: toward an account of vowel normalisation. Lang Cogn 25(6):808–839. https://doi.org/10.1080/01690965.2010.490047
Mumm CAS, Urrutia MC, Knörnschild M (2014) Vocal individuality in cohesion calls of giant otters, Pteronura brasiliensis. Anim Behav 88:243–252. https://doi.org/10.1016/j.anbehav.2013.12.005
Owings DH, Morton ES (1998) Animal vocal communication: a new approach. Cambridge University Press. https://doi.org/10.2307/1370020
Puts DA, Hodges CR, Cárdenas RA, Gaulin SJ (2007) Men’s voices as dominance signals: vocal fundamental and formant frequencies influence dominance attributions among men. Evol Hum Behav 28:340–344. https://doi.org/10.1016/j.evolhumbehav.2007.05.002
R Development Core Team (2021) R foundation for statistical computing. http://www.R-project.org
Reby D, McComb K (2003) Anatomical constraints generate honesty: acoustic cues to age and weight in the roars of red deer stags. Anim Behav 65:519–530. https://doi.org/10.1006/anbe.2003.2078
Reby D, McComb K, Cargnelutti B, Darwin C, Fitch T, Clutton-Brock TH (2005) Red deer stags use formants as assessment cues during intrasexual agonistic interactions. Proc R Soc B 272:941–947. https://doi.org/10.1098/rspb.2004.2954
Reby D, Wyman MT, Frey R, Charlton BD, Dalmont JP, Gilbert J (2018) Vocal tract modelling in fallow deer: are male groans nasalized? J Exp Biol 221:jeb179416. https://doi.org/10.1242/jeb.179416
Rendall D, Rodman PS, Emond RE (1996) Vocal recognition of individuals and kin in free-ranging rhesus monkeys. Anim Behav 51:1007–1015. https://doi.org/10.1006/anbe.1996.0103
Rendall D, Owren MJ, Rodman PS (1998) The role of vocal tract filtering in identity cueing in rhesus monkey (Macaca mulatta) vocalizations. J Acoust Soc Am 103:602–614. https://doi.org/10.1121/1.421104
Root-Gutteridge H, Ratcliffe VF, Korzeniowska AT, Reby D (2019) Dogs perceive and spontaneously normalize formant-related speaker and vowel differences in human speech sounds. Bio Lett 15:20190555. https://doi.org/10.1098/rsbl.2019.0555
Searby A, Jouventin P (2003) Mother-lamb acoustic recognition in sheep: a frequency coding. Proc R Soc B 270:1765–1771. https://doi.org/10.1098/rspb.2003.2442
Seyfarth RM, Cheney DL (2010) Production, usage, and comprehension in animal vocalizations. Brain Lang 115:92–100. https://doi.org/10.1016/j.bandl.2009.10.003
Sharp SP, Hatchwell BJ (2005) Individuality in the contact calls of cooperatively breeding long-tailed tits (Aegithalos caudatus). Behaviour 142:1559–1575. https://doi.org/10.1163/156853905774831918
Smith DR, Patterson RD, Turner R, Kawahara H, Irino T (2005) The processing and perception of size information in speech sounds. J Acoust Soc Am 117:305–318. https://doi.org/10.1121/1.1828637
Sueur J, Aubin T, Simonis C (2008) Seewave, a free modular tool for sound analysis and synthesis. Bioacoustics 18:213–226. https://doi.org/10.1080/09524622.2008.9753600
Taylor AM, Reby D (2010) The contribution of source–filter theory to mammal vocal communication research. J Zool 280:221–236. https://doi.org/10.1111/j.1469-7998.2009.00661.x
Taylor AM, Reby D, McComb K (2010) Size communication in domestic dogs (Canis familiaris) growls. Anim Behav 79:205–210. https://doi.org/10.1016/j.anbehav.2009.10.030
Torriani M, Vannoni E, McElligott AG (2006) Mother–young recognition in an ungulate hider species: a unidirectional process. Am Nat 168:412–420
Vannoni E, McElligott AG (2007) Individual acoustic variation in fallow deer (Dama dama) common and harsh groans: a source-filter theory perspective. Ethology 113:223–234. https://doi.org/10.1111/j.1439-0310.2006.01323.xc
Vannoni E, McElligott AG (2008) Low frequency groans indicate larger and more dominant fallow deer (Dama dama) males. PLoS ONE 3:e3113. https://doi.org/10.1371/journal.pone.0003113
Vignal C, Mathevon N, Mottin S (2004) Audience drives male songbird response to partner’s voice. Nature 430:448–451. https://doi.org/10.1038/nature02645
Wanker R, Fischer J (2001) Intra- and interindividual variation in the contact calls of spectacled parrotlets (Forpus conspicillaus). Behaviour 138:709–726. https://doi.org/10.1163/156853901752233361
Acknowledgements
We are grateful to Chiara Calcari, Eleonora Cresta, and Elena Fumagalli for recording the contact calls used in the playback experiments and to Veronica Clerico for scoring the videos used for the inter-observer agreement. We also thank Cristina Pilenga and Francesca Bandoli for providing access to the African penguins at Zoomarine Roma and the Zoological Gardens of Pistoia, respectively. We thank Martina Tubito and all the penguin keepers of Zoom Torino (www.zoomtorino.it) for their excellent support.
Funding
Open access funding provided by Università degli Studi di Torino within the CRUI-CARE Agreement. LB and FT were supported by the University of Turin through a MIUR postdoctoral fellowship and a PON PhD scholarship REACT-EU FSE DM 1061, respectively.
Author information
Authors and Affiliations
Contributions
Conceptualisation: LB, LF. Formal analysis and investigation: CM, FT, LB, VI. Writing—original draft preparation: FT, LB, LF. Writing—review and editing: all authors. FT prepared Fig. 1. LB prepared Fig. 2 and Tables 1, 2. Resources: LF, VI, MG.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The Ethics Committee of the University of Turin approved the study (approval number 280324). All procedures also followed the guidelines for the treatment of animals in behavioural research and teaching (ASAB 2022).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 46550 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Terranova, F., Baciadonna, L., Maccarone, C. et al. Penguins perceive variations of source- and filter-related vocal parameters of species-specific vocalisations. Anim Cogn 26, 1613–1622 (2023). https://doi.org/10.1007/s10071-023-01806-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-023-01806-w