Abstract
Functional foods have become an essential element of the diet in developed nations, due to their health benefits and nutritive values. Such food products are only called functional if they, “In addition to basic nutrition, have valuable effects on one or multiple functions of the human body, thereby enhancing general and physical conditions and/or reducing the risk of disease progression”. Functional foods are currently one of the most extensively researched areas in the food and nutrition sciences. They are fortified and improved food products. Presently, probiotics are regarded as the most significant and commonly used functional food product. Diverse probiotic food products and supplements are used according to the evidence that supports their strength, functionality, and recommended dosage. This review provides an overview of the current functional food market, with a particular focus on probiotic microorganisms as pivotal functional ingredients. It offers insights into current research endeavors and outlines potential future directions in the field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last few years, the field of food production has been significantly transformed due to consumer demand. Consumers’ belief that food has a direct impact on well-being, and health has significantly contributed to a change in the field of food production. Nowadays, in addition to hunger satisfaction, food provides essential nutrients, prevents illnesses, and aids in the progression of consumers’ mental and physical health (Menrad, 2003). Functional foods play a remarkable contribution in this context. The need for this kind of food has increased considerably due to the high cost of medical centers, improving quality of life, and increasing life expectancy (Kotilainen et al., 2006). During the 1980s, the concept of functional foods originated in Japan (Valdemiro Carlos, 2011) which provides basic nutrition essential for living (Alissa and Ferns, 2012), helps in the welfare of physical and mental health, provides treatment and prevention from certain diseases, and enhances physiological functions (Lobo et al., 2010) like improving systemic circulation, immuno-potentiation, and aging control (Al-Sheraji et al., 2013). Functional food is defined as, “Food products that show resemblance to traditional food, but modified to have added health benefits to human and are consumed as a part of normal diet”.
Back in the earlier times, the advancement of functional food relied on food products strengthened with added minerals or/and vitamins, such as vitamin C, vitamin E, folic acid, calcium, iron, and zinc (Sloan, 2000). Later, the emphasis shifted to food products strengthened with added micronutrients like, phytosterol, soluble fiber, and omega-3 fatty acids to encourage better well-being or for the prevention of certain diseases like cancers (Sloan, 2002). Currently, further steps have been taken by food companies for the progression of new functional foods that provide multiple benefits to human health in one food product (Sloan, 2004). The physiological benefits of functional foods include, modification of the immune system, improving heart health, improved gastrointestinal system, healthy urinary tract system, reduce blood pressure, anti-inflammatory properties, antiviral and antibacterial activity, protection of vision, decreased osteoporosis, and reducing obesity. The functional property of these foods is based on biologically active components that are naturally present in the product and require optimizing health benefits by adding a particular ingredient. Recently, probiotics have acquired public attention due to their potential to improve human health. Due to this demand, the probiotic market has expanded rapidly worldwide. And as a result, they are used as supplements and as functional food raw materials (Chen et al., 2023), Table 1 represents major probiotic strains used in the market (Anwer et al., 2019; Ballini et al., 2023). This review will focus on the development of functional foods supplemented with probiotics, demonstrate the properties of probiotics, and cover all the possible treatment strategies currently in practice using probiotics by linking them with ongoing clinical studies. The future directions of probiotic functional food will also be discussed.
Probiotics as a functional food
The notion of probiotics was established by scientist Elie Metchnikoff during the 1900s. He discovered that consuming live microbes such as Lactobacillus bulgaricus available in yogurt or fermented milk show improvement in several gastrointestinal (GIT) properties (De Simone, 2019). Recently, probiotics are defined as “living microbes which confer a health benefit to the host, upon ingestion in a tolerable amount” by the World Health Organization (WHO) and the Food and Agricultural Organization (FAO) of the United Nations (Hill et al., 2014). According to International Association for Scientific Prebiotics and Probiotics (ISAPP), certain minimum requirements must be fulfilled by probiotic microorganisms to be included in the category of functional food. Criteria such as evidence about their genus, species, and strain identifications; a valid scientific nomenclature for designation of strain; being deposited in an international culture collection; and having health benefits that must be validated by human study (at least one). Furthermore, a probiotics-containing functional food product must contain enough live microorganisms to offer the demanded positive health effects until the expiry date of the food product (Fusco et al., 2022). Moreover, probiotics should be adaptable to adverse GIT conditions, exhibit antagonistic effects against pathogenic microorganisms, have proven health benefits on the host, stimulate the immune system, and maintain their potency when exposed to certain food processing conditions (Plaza-Diaz et al., 2019). Bifidobacterium and Lactic acid bacteria are the most commonly studied and extensively used bacteria in the area of probiotics, however some different genera, for instance, Streptococcus, Enterococcus, Saccharomyces, and Bacillus have also been commercialized (Sarao and Arora, 2017). One of the most studied genera is Lactobacillus, and the most recent update reported 23 novel genera of Lactobacillus. This has sparked a new wave of scientific inquiry and their role as probiotics needs to be further explored and verified (Zheng et al., 2020). They are the normal flora of the colon. Microflora present in the colon performs several unique functions, and evaluating these functions is essential (Roberfroid et al., 1995). For the testing and development of functional foods, the gut is an obvious and natural target since it operates as a midway connection between metabolic pathways and the diet of human. For application in the dairy industry, an ideal probiotic must be acid and bile-resistant, perform modulation of the immune system, attach to epithelial cells of humans, produce antimicrobial compounds, grow exponentially, and produce health benefits to humans (Gorbach and Newton, 1996). Though the mechanisms of action of probiotics are specific to certain strains and it is diverse (Hill et al., 2014; Plaza-Diaz et al., 2019). Certain prebiotic substances serve as a stimulant for the growth of probiotics in the gut. Prebiotics like lactitol, inulin, lactulose, and xylitol are currently on the market due to their claimed benefits to boost the effect of probiotics (Zubillaga et al., 2001).
Global statistics and therapeutic dosage
Currently, functional food is made up of vitamins, minerals, probiotics, and prebiotics which are now in human use in the form of certain products like yogurts, fermented milk baby foods, sports drinks, chewing gum, and confectionery. More recently, gut health functional food products particularly probiotics has dominated the food market in Europe and Japan with the launch of 379 products globally in 2005, several examples of commercial probiotic products with their origin are listed in Table 2 (Kaur and Das, 2011; Siró et al., 2008; Vergari et al., 2010). The growing interest of people in lifestyle and health, as well as issues associated with digestive and metabolic disorders, are essential factors in the contribution to expanding the probiotics industry (Elshaghabee et al., 2017), which accounts for a significant portion of the functional food market (Sarao and Arora, 2017). The global probiotics market in 2019 had a value of $48.4 billion and is predicted to rise at a compound annual growth rate (CAGR) of 7.4% from 2019 to 2024 (BBC, 2020). Asia now has the largest market for probiotic products, as well as the highest growth rate (Intelligence, 2019), indicating that there are intriguing demands to be investigated (Kwak, 2014). The practice of using probiotics in food products or as a supplement having a particular health benefit requires prior human experiments, such as randomized clinical trials, positive meta-analyzes, or a strong suggestion from observational research studies (Hill et al., 2014). The recommended amount of probiotic microorganisms varies depending on the strain and product, stated by World Gastroenterology Organization (WGO) (Organisation, 2017). Even though numerous commercially accessible products contain a probiotic dose in the range of 1 to 10 billion colony-forming units (CFU), few formulations have been demonstrated to be effective at a low level of dose, whereas others need higher doses (Sarao and Arora, 2017). Upon regular intake of 100 mL or 100 g of probiotic food, it is advised that probiotic formulae should have at least 106–107 CFU per gram of probiotic food, otherwise a total of 108–109 CFU, to have the therapeutic potential (Flach et al., 2018).
Health benefits of probiotics
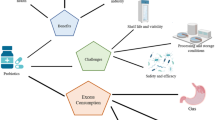
Major health benefits produced by probiotic microorganisms are the establishment of favorable conditions of the intestine, a strong immune system, and a digestive system. Figure 1 demonstrates the different health benefits of probiotics. Probiotics have been shown to avert several inflammatory and allergic disorders (like rhinitis and atopic dermatitis), reduce the incidence of diarrhea, serve as antibiotics, control infections, and protect against bladder and colon cancers (De Prisco and Mauriello, 2016; Plaza-Diaz et al., 2019). Probiotics have been linked to prevent and/ or treat acute diarrheal infections, necrotizing enterocolitis, antibiotic-related diarrhea, pediatric colic, allergies, lactose intolerance, ulcerative colitis, Helicobacter pylori infection, Crohn’s disease, metabolic illnesses, neurological diseases, and respiratory tract infections (Liu et al., 2018; Télessy, 2019). Numerous clinical trials using probiotics have been successful to treat or alleviate symptoms of several diseases, for example, irritable bowel infection (Shadnoush et al., 2015), irritable bowel syndrome (Yoon et al., 2015), obesity (Song et al., 2020), infantile colic (Chau et al., 2015), Parkinson’s (Barichella et al., 2016), diabetes (Soleimani et al., 2017), rheumatoid arthritis (Zamani et al., 2016). Furthermore, in a study, Lactobacillus rhamnoses were encapsulated in a hydrogel that contains thiolated hyaluronic acid to treat Salmonella-induced enteritis. It was found that the encapsulated probiotic was more effective than free cells, suggesting that it could be a viable alternative to antibiotics (Xiao et al., 2020). Probiotics are widely in use among functional food products due to their proven health benefits and alternate treatment options for many diseases, some of which will be discussed in detail in this review.
Impact on the age group
The fecal flora of children is different than in adults, disease such as gastroenteritis can alter the bacterial profiles in children. The frequency of dysbacteriosis is particularly high in children after antibiotic therapy. Probiotic functional foods can help to restore the microbial equilibrium of the intestine and control the side effects of antibiotic therapy. Adults, particularly older people frequently develop a condition called, atrophic gastritis, also called the incapability to secrete stomach acid. In the United States, almost one-third of older people are being affected by this condition. In atrophic gastritis, a lack of gastric acid can lead to the overgrowth of bacteria in the small intestine, which can influence micronutrient absorption (Zubillaga et al., 2001). The functional foods containing probiotics have the potential to regulate intestinal bacterial balance and enhance health outcomes within various demographic groups.
Gastrointestinal (GIT) disorders
Allergy to food
Several research findings suggested that probiotic bacteria (Lactobacillus rhamnosus GG) may increase the mechanisms of endogenous barriers in individuals having food allergies and atrophic dermatitis. By reducing colonic inflammation, probiotics may be effective in the treatment of food allergies (Majamaa and Isolauri, 1997). When administering novel probiotic functional foods for treating GIT problems, the antigenicity level of the diet should be considered. Probiotics like Lactobacillus rhamnosus GG (LGG) can not only correct abnormal transport of macromolecules, but can also have a positive result on mucosal breakdown depending on the dietary antigen (Pessi et al., 1998). Functional food made of LGG is already available on the market and has proven health benefits, but it is imperative to conduct further evaluations to ensure the safety of these probiotics.
Diarrheal illness
It has been shown that pathogenic microorganisms such as S. typhosa, S. dysenteriae, and E. coli are inhibited by a fermented probiotic product comprising L. acidophilus. Probiotic administration in bacterial diarrhea produces a favorable impact by the production of antimicrobial compounds by L. acidophillus, that neutralize the enterotoxins produced by E. coli (Rani and Khetarpaul, 1998). L. acidophilus and bifidobacteria were found to reduce the development of colonic flatus in humans and the modulation of diarrhea caused by Clostridium difficile (Gallaher et al., 1996). Nine randomized and placebo-controlled trials have been conducted around the world for the prevention of diarrhea with different probiotic species, including, Bifidobacterium lactis, Lactobacillus acidophilus Lactobacillus GG, Streptococcus thermophilus, Lactobacillus reuteri, and Lactobacillus rhamnosus. They have demonstrated dose dependent efficacy to treat diarrhea (Guandalini, 2011). In another study, idiopathic diarrhea was improved by using probiotic Escherichia coli Nissle 1917 (EcN) (Rudinsky et al., 2023).
Lactose malabsorption
Lactobacillus supplementation is thought to improve lactose fermentation and alleviate lactose intolerance symptoms. According to studies, consumption of lactobacilli-containing food products can lower the activity of fecal bacterial enzymes such as nitroreductase, beta-glucuronidase, and azoreductase. Lactose malabsorption is adapted fast and metabolized in the small intestine by colonic flora. The study revealed that L. acidophilus improves in-vitro lactose fermentation when adaptation to lactose load by colonic flora is not developed and a stable bacterial population is not maintained. Lactose digestion is improved by Lactobacillus acidophilus strain LA-1, suggesting that the metabolic changes caused by supplements having lactobacilli can occur when a stable microflora has not been formed (Zubillaga et al., 2001). There are functional foods made of Lactobacillus acidophilus currently in human use.
Helicobacter pylori (H. pylori) infection
Helicobacter pylori is spiral-shaped and gram negative pathogen responsible for causing peptic ulcer and gastric malignancies (Patel et al., 2014). The association of diet with the occurrence of peptic ulcers has been linked. It has been demonstrated that people with confirmed peptic ulcers, showed a lower consumption level of fermented milk products and vegetables and a higher consumption level of bread, meat, and milk than ordinary individuals (Elmståhl et al., 1998). The lack of Lactobacillus specie in the stomach was discovered in patients with H. pylori infection. A decline in the Bifidobacteria population and an increase in opportunistic enterobacteria, as well as changes in local immunity, were found in H. pylori-infected population. The results suggested that using probiotic functional food which includes lactobacilli and bifidobacteria generated good outcomes for the treatment of immunological and microecological abnormalities. In children, H. pylori associated with gastroduodenal pathology can be treated with probiotics containing bifidobacteria and lactobacillus along with triple antibacterial therapy. The probiotic formulation is prescribed in the early phase of etiotropic therapy (Lykova et al., 1999). Based on many clinical studies, administration of probiotics combined with standard antibiotic therapy effectively treats H. pylori infection. Research reported that the use of Lactobacillus casei, Lactobacillus rhamnosus GG, Lactobacillus reuteri, and Saccharomyces boulardii can efficiently eliminate H. pylori infection (Keikha and Karbalaei, 2021). Probiotics are extensively used in therapeutic purpose for H. pylori infection as demonstrated in Table 3. However, the focus of future studies should be on probiotic species, optimal dosage, formulation in functional food, and length of treatment.
Immunomodulation
Probiotics have immunomodulatory properties which are strain specific. A study demonstrated that the phagocytic capability of the leucocytes isolated from blood samples of individuals who had previously consumed probiotics was boosted by L. acidophilus La1, which was consistent with the adhesion potential of the bacterium. However, even Bifidobacterium lactis Bb12, which has a somewhat lower adhesion, has been demonstrated to significantly improve phagocytosis. Furthermore, probiotics can stimulate IgA production by B cells. A noteworthy rise of IgA concentration in serum was observed in the subjects of the study with the intake of fermented milk containing L. acidophilus La1 and Bifidobacterium bifidum, following Salmonella typhi Ty21 vaccination. Additionally, children who received rotavirus vaccination and consumed L. rhamnosus GG alongside presented an increased quantity of IgA-secreting cells (Delcenserie et al., 2008). Probiotics are classified as either ‘immunostimulatory’ or ‘immunoregulatory,’ based on their capability to produce IL-12, hence augmenting the defense system of a host via the enhanced activity of NK cell and TH1 pathways, or ‘immunoregulatory,’ based on their capability to stimulate IL-10 and pathway of T-cell regulation. Lactobacilli often fall into the category of immunostimulatory, while Bifidobacterium generally falls into the category of immunoregulatory (Yaqoob, 2014). In another study, Bifidobacterium breve was used in the treatment of severe combined immunodeficient (SCID) mice, T cell-derived IL-10 inhibited T cell-dependent intestinal inflammation in mice (Jeon et al., 2012). In future their effect must be evaluated in well-designed clinical research.
Anti-cancer activity
Cancer is the deadly disease worldwide. The administration of probiotic supplementation has been proven in animal trials to prevent the formation, development, and metastasis of chemically induced and transplantable tumors. Probiotic therapy has also been shown to decrease the occurrence of colonic cancer by preventing the transformation of procarcinogens to active carcinogens, production of antimutagenic compounds, inactivation of mutagenic compounds, suppression of pro-carcinogenic bacterial growth, immune function enhancement, reducing mutagens absorption of from the intestine, anti-proliferation by cell differentiation and the regulation of apoptosis, undigested food fermentation which aids in generating short-chain fatty acids (SCFA), and inhibiting signaling pathways for tyrosine kinase (Gill and Guarner, 2004; Uccello et al., 2012). The results of many clinical studies indicate the effectiveness of probiotics in prevention and treatment of many cancers including breast, cervical, colon, liver, gastric, pancreatic, bladder, and colorectal cancer (Table 4).
Hypercholesterolemia
Hypercholesterolemia is defined as metabolic syndrome associated with abnormal serum and cellular cholesterol levels (Kumar et al., 2012). Cholesterol is significant biological compound, but the high serum cholesterol levels have a direct role in many health disorders, such as, hypertension, coronary heart disease, diabetes type II, atherosclerosis (Chien et al., 2010). Existing evidence suggests that the management of cholesterol decreases the chance of cardiovascular disease or reduces its progression. Previously, reducing the level of low-density lipoprotein (LDL) cholesterol in plasma was restricted to changes in diet and the use of medications such as statins. Recently, probiotic functional food are in the growing demand for the control of cholesterolemia (Poli et al., 2018). Daily administration of L. rhamnosus GG in a concentration of 1 × 108 CFU per mouse, for 13 weeks in dyslipidemic mice helped to restore the microbiota of the gut, and showed improvement in hypercholesterolemia, hepatic fat accumulation, and hypertriglyceridemia (Kim et al., 2016). In another study, L. rhamnosus hsryfm1301 induced for 28 days in 109 CFU/mL, reduced the level of cholesterol and triglycerides in the serum of a hyperlipidemic rat model (Chen et al., 2014). Similarly, consumption of L. acidophilus NS1 for 10 weeks in a concentration of 1.0 × 108 CFU/mL, on a long-term daily basis reduced LDL-cholesterol, plasma cholesterol, and triglycerides levels in a diet-induced obese model of mice (Song et al., 2015). Moreover, in diet-induced hypercholesterolaemic rats, a daily intake of a mixture of probiotics containing B. lactis, B. longum, B. brevis, L. plantarum, and L. reuteri in different doses for 8 weeks, lowers the LDL-cholesterol, total serum cholesterol, triglycerides, and prevent hepatic steatosis (Kim et al., 2017). Clinical findings on a controlled, randomized, double-blind trial suggest that L. plantarum CECT 7527, CECT 7528, and CECT 7529 supplementation for 12 weeks decreases the level of cholesterol in hypercholesterolaemic patients (Fuentes et al., 2013). Furthermore, L. acidophilus L1 and probiotic yogurt made by fermentation with a starter culture of B. lactis and L. acidophilus, when supplemented with hypercholesterolemic subjects showed a reduction in cholesterol level (Anderson and Gilliland, 1999; Ataie-Jafari et al., 2009). Recently, a meta-analysis with clinical trials confirmed that consumption of fermented milk products supplemented with probiotics and supplements containing probiotic bacteria effectively reduces LDL-cholesterol and total cholesterol levels in serum (Shimizu et al., 2015). Likewise, another study proved that ingestion of probiotics affects reducing LDL-cholesterol and total cholesterol in human subjects with high cholesterol, borderline high levels, and a normal level of cholesterol (Guo et al., 2011). Probiotic functional foods play a significant role in regulating lipid metabolism and have a therapeutic effect on dyslipidemic disorders. Further large-scale studies on clinical subjects must be conducted.
Diabetes mellitus type 2 (DMT2)
Diabetes mellitus Type 2 is a common metabolic illness, and it occurs when the pancreatic cells are unable to produce sufficient insulin for maintaining a normal level of glucose in the blood, or when the pancreatic cells become resistant to insulin (Boada and Martinez-Moreno, 2013). Associated risk factors are age, physical inactivity, diet, obesity, genetic susceptibility, and sedentary lifestyle (de Almeida-Pititto et al., 2015; Hu et al., 2001). Current evidence proposes that disturbance of intestinal permeability and dysregulation of gut microbiota could also be linked to DMT2 development (Cani et al., 2012; Flint et al., 2012; Gerritsen et al., 2011; Navab-Moghadam et al., 2017). Disruption in gut microbiota cause changes that disrupt pancreatic cells, reduce insulin resistance, and develop DMT2 (Ly et al., 2017). Studies suggest that probiotic formulations can restore gut permeability and microbial balance. They can also reduce pro-inflammatory markers, reactive oxygen species, and insulin resistance (Wang et al., 2017a, 2017b). An experimental finding indicates that long-term intake of 0.1 g probiotic lyophilized powder having Bacillus subtilis, L. reuteri, and L. crispatus for 8 weeks in the concentration of 1010 CFU/mL/day, reduced the HbA1c and plasma glucose levels. It also improved the oral glucose tolerance test and in adipose tissues, Glut-4 mRNA is up-regulated in the streptozotocin-induced diabetic rats (Memarrast et al., 2017). Moreover, consuming a mixture of probiotics having L. plantarum, L. rhamnosus, L. casei, L. plantarum CCFM36, and L. breve for 10 weeks with a dose of 1.6 × 1010 CFU/day, efficiently decreased leptin levels and HbA1C, enhanced insulin resistance, glucose tolerance and protected against the pancreatic impairment in diabetes type 2 mice (Li et al., 2016). Human studies in placebo-controlled, small-scale, double-blind groups have also revealed the therapeutic impact of the probiotic product on DMT2. Another meta-analysis in a double-blind, randomized placebo-controlled trial for 12 weeks was carried out, probiotics were supplemented in a dose of 3 × 1010 CFU. Six viable cell probiotics including L. casei, L. acidophilus, L. lactis, B. longum, B. bifidum, and B. infantis were administered to 136 patients with no insulin-dependent diabetes separated into a probiotic group (37 males & 31 females) and a placebo group (34 males & 34 females). It improved fasting glucose levels and serum HbA1c in both genders Recent meta-analyses of randomized clinical studies supported the results and confirmed that the probiotic formulation is efficiently related to an improved HbA1c level and fasting insulin in DMT2 patients(Akbari and Hendijani, 2016). Another 8 weeks of randomized controlled clinical findings demonstrated improved factors related to oxidative stress, for example, glutathione reductase and glutathione peroxidase levels in 48 patients with diabetic kidney disease. They were administered a 200 mL L. plantarum A7 per day probiotic with soy milk (Miraghajani et al., 2017). Furthermore, in a different clinical study, multi-probiotic species were administrated containing freeze-dried and viable strains of L. bulgaricus, L. casei, L. acidophilus, L. rhamnosus, B. longum, B. breve, and Streptococcus thermophilus in different concentrations for 8 weeks, in association with 100 mg fructo-oligosaccharide in Iranian diabetic patients. It showed improvement in the total antioxidant capacity and total glutathione levels (Asemi et al., 2013). The outcome of these findings makes probiotics a perfect therapeutic candidate to be involved in functional food.
Chronic kidney disease
Renal impairments can cause cardiovascular disorders through several mechanisms. Several factors that cause renal abnormality are traditional factors such as hypertension, diabetes, and dyslipidemia. The non-traditional factors include hemodynamic and metabolic abnormalities caused by a renal abnormality (e.g., oxidative stress and inflammation) that can impact the development of cardiovascular disorders in patients with chronic kidney disease (Longenecker et al., 2002). Research has found that the composition of microbiota in patients with chronic kidney disease is altered, and the communication between gut microbiota and the host is a key event in the pathophysiology of the disease. Whereas uremia impacts both the composition and metabolism of gut microbiota, significant uremic toxic compounds are produced by the metabolism of gut microbiota, suggesting that this process is bi-directional. These toxins are eliminated by the kidneys, primarily through tubular secretion, and are hence classified as uremic toxins (Poesen et al., 2013). Intestinal bacteria digest about 10 g of protein every day in the colon, converting it to metabolites like ammonium, amines, phenols, indoles, and thiols. Most of these products are removed through feces; however, the kidneys are responsible for a portion of their absorption and disposal. The accumulation of these products is caused by impaired renal function, as seen in CKD (Evenepoel et al., 2017; Khoury et al., 2017). Moreover, disruption of the gut microbiota damages the epithelial barrier integrity, which has been linked to gut ischemia and intestinal wall edema, the result is increased endotoxin exposure of the host tissues, together with the kidneys (Vaziri, 2012). Emerging approaches to restoring the intestinal environment during the progression of CKD include the administration of probiotics (Koppe et al., 2015). Genetically modified microencapsulated live cells from the urease-producing bacteria E. coli DH5 were orally administered to uremic rats and significantly decreased the plasma urea levels (Prakash and Chang, 1996). In a different study, a casein-based food diet with added probiotics Sporlac (L. sporogenes, 1 × 108 CFU per day), Bacillus pasteurii (1 × 109 CFU/day), kibow cocktail (containing Bifidobacterium spp., L. acidophilus, L. bulgaricus, L. reuteri, L. casei, S. thermophilus at 1 × 1010 CFU/day) or S. boulardii (1 × 109 CFU/day) was fed to a chronic renal failure model (nephrectomized rats) for 16-weeks trial and reduced blood urea nitrogen (BUN) levels and extended life were observed (Ranganathan et al., 2005). Clinical studies found that the intake of L. acidophilus orally decreased the levels of intestinal toxins such as nitroso-dimethylamine, and dimethylamine (DMA), which are produced in the plasma of patients on hemodialysis (Simenhoff et al., 1996). The results were supported by another study in which, a double-blind pilot study was conducted on 46 diseased individuals in stage III or IV of chronic kidney disease. A mixture of probiotics named KB (B. longum KB31, L. acidophilus KB27, and S.thermophilus KB19, in a dose of 109 CFU per day) was given for a duration of six months. A reduction in BUN was observed together with improved quality of life (Ranganathan et al., 2010). A clinical trial on stages 3 and 4 of disease patients was carried out and a commercially available lyophilized symbiotic named Probinul-neutro®, containing L. casei subsp. Rhamnosus, L. plantarum, L. gasseri, L. acidophilus, L. sporogenes, L. salivarius, B. longum, B. infantis, and Streptococcus thermophilus was given, along with prebiotic inulin tapioca-resistant starch was suggested for 4 weeks in the dosage of 5 g × 3/day. This supplementation reduced the level of plasma p-cresol (Guida et al., 2014). This indicates the therapeutic potential of probiotics in chronic kidney disease patients but there is no defined dosage, intervention timeframe, mechanism, and pathways to define probiotic efficacy in this regard. So, further research is needed to explore the full potential of probiotics in reducing chronic kidney disease (Neto et al., 2018).
Heavy metal intoxication
The typical treatment method against toxicity produced by heavy metals was dependent on chelation therapy, which uses different chemical chelators. Chelators have several side effects, including cardiac arrest, kidney overload, anemia, and mineral insufficiency (Flora and Pachauri, 2010). Recently, probiotics, nanoparticles, essential amino acids, folate, and vitamins C and E have all been used as treatment candidates for heavy metal intoxications (Duan et al., 2020; Inbaraj and Chen, 2012; Yang et al., 2019).
New functional food was proposed in a study composed of selenium nanoparticles (SeNPs) generated by the means of green synthesis utilizing L. casei. For the first time, its capacity was investigated for liver injury bioremediation. The ability of SeNPs to defeat cadmium-induced hepatic toxicity was also checked. Elemental SeNPs exist in two forms; purified SeNPs and lactic acid bacteria (L. casei) together with endogenous SeNPs (called LSeNPs). They were investigated in mice with liver toxicity induced by cadmium. LSeNPs were given orally for 30 days in a dosage of 0.4 mg/kg b.w. Treatment with both SeNPs forms ameliorated Cd-induced liver damage in the mouse. Results presented a decrease in pro-apoptotic bax, including a rise in the expression of anti-apoptotic bcl-2. Also, a reduction in the gene expression of inflammatory markers of the liver was observed. LseNPs showed excellent hepatoprotective effects. A functional diet containing both elemental SeNPs and probiotic bacteria might be used to eliminate liver damage caused by cadmium and improve the nutritional potential and health benefits. A potential new technology for the food industry is to create yogurt enhanced with LSeNPs with heavy metal remediation properties (Vicas et al., 2021). Further clinical studies must be determined to support the effectiveness of this in functional food products.
Microencapsulation of probiotics
Most of the functional foods formulated with probiotics are available in the dairy industry. Many available foods in the market that are incorporated probiotics are supplemented with the free microbe, and just a few use their microencapsulated forms (De Prisco and Mauriello, 2016). Besides their function in boosting the health of individuals upon intake, probiotics could also be integrated into edible polymeric matrices to produce bioactive packaging of food. They can substitute antibiotics by suppressing spoilage and infective bacterial species, also improving the safety of food (Espitia et al., 2016). The key functional food products having probiotic microcapsules are bakery products like cakes, bread, and biscuits; Dairy products like cheese and yogurt; vegetables and fruit juices; meat products like fermented sausages; and others include mayonnaise, ice cream, and fermented beverages. Table 5 shows several examples of microencapsulated probiotic bacterial species and the method used for encapsulation in food products (Burgain et al., 2011; De Prisco and Mauriello, 2016; Terpou et al., 2019). Probiotic bacteria can be co-encapsulated with biologically active materials such as prebiotics, antioxidants, curcuminoids, and omega-3 fatty acids within the same matrix and subsequently integrated into food, making it an afunctional product. The bioactivity of both is increased and it can also show synergistic health benefits to the host. Appropriate wall materials are required to achieve such synergistic effects, and their functioning must be improved (Misra et al., 2021). Functional products of these types are generally commercialized in powdered form, chewable tablets, or capsules. The process of encapsulation could be achieved through a technique called “spray coating”. In this technique, fat-based polymers are sprayed on a probiotic microorganism to coat them, which increases their availability in the environment of the gastrointestinal system. Commercialized products are Probiocap™ and STAR™ technologies (Kwak, 2014). L. casei was added to juices of citrus fruits in the free form and microencapsulated form by using the vibration technique. Their viability was evaluated at 4 °C after 28 days based on the fruit juice type, the low pH had an impact on bacterial survival during storage. A few microcapsules started to break in the pineapple juice, but they showed recovery after 28 days with 100% viability (2.3 × 107 CFU/g spheres). The orange juice showed greater than 90% viability (5.5 × 106 CFU/g spheres). Though, raspberry juice showed a rapid drop in viability, eventually vanishing at the end of storage, possibly due to the absorption of anthocyanin inside microcapsules. Another study demonstrated the protection of thermotolerant lactic acid bacteria against pasteurization in cooked sausages, ionotropic alginate-pectin gels were used to co-encapsulate them with the prebiotics produced by residues from agriculture and industry (Barragán‐Martínez et al., 2020). Sausages had good sensory qualities, a higher count of lactic acid bacteria, the pathogenic bacteria were inhibited, and the synergetic prebiotics use reduced oxidative rancidity of fat (Reque and Brandelli, 2021).
In the most recent studies, the spray drying technique was used to prepare a powdered functional food made up of carbohydrate polymers and active ingredients. Bacillus clausii as a probiotic, and Quercetin as an antioxidant were co-microencapsulated using carrying agents; inulin (IN) and maltodextrin (MX). The IN-MX blends had a synergistic effect on viability and antioxidant activity. The microbial viability improved by adding MX, whereas antioxidant activity was improved by adding IN. A surface response plot revealed that the yield was highly dependent on temperature drying and then the concentration of IN. This study demonstrated the benefits of using combinations of carbohydrate polymers for the preservation and microencapsulation of active compounds for applications in functional foods and pharmaceutical use (Saavedra-Leos et al., 2022). The use of probiotics in functional food via the microencapsulation technique has a plethora of benefits because of its advantage in protecting a functional component. It can help in reducing the global shortage of micronutrients and has a promising future (Palanivelu et al., 2022).
Discussion
Functional foods are by far the most rapidly growing division of the food and beverage industry with a promising future. Intestinal microbiota research is gaining attention in a variety of fields. Various research studies give evidence for the use of probiotics in food for obtaining health advantages. The significance of using gut microorganisms in the production of functional foods is a major area of research. The evidence from scientific data suggested that disturbance of the gut microbial environment leads to multiple diseases, and intake of functional food based on probiotics demonstrated beneficial health effects in so many ways. However, there are still some knowledge gaps in most of the studies as there is limited information on the safety of probiotics in many pieces of research. More evidence is required for the survival of probiotics in functional food for the duration of its shelf life. Furthermore, food scientist needs to focus on the selection of the most appropriate strain for the combination of probiotics in the food matrix, which is an essential component. Also, convenient packaging and storage conditions should be provided to produce effects in the host, this area should be explored deeply. After a deep evaluation of studies conducted on the properties of probiotics, the concluding results are still controversial, and less research has been carried out on human subjects. Further research on the assessment of probiotics in the prevention of diseases is a need and more large-scale clinical research must be performed. Finally, the area of consumer awareness must be focused on to achieve maximum success in the probiotic functional food market.
Future direction
Functional food is currently involved in extensive scientific research to fully explore this area of study. Globally, government sectors, and academic, and private research institutions are dedicating significant efforts to identify how food ingredients and functional foods might help prevent disease and/or infection, improve health, lower the cost of healthcare, and enhance the standard of health for people. Nutrigenomics is a developing field in which the link between diet and disease development is analyzed depending on a genetic profile of an individual. In this, a personalized functional diet can be prescribed using an individual’s exact genetic profile. It is going to have a significant impact on the future of new probiotic functional food products for the prevention of disease (Hasler, 2002). The next-generation probiotics shall be based on population-level strategy and/ or personalized nutrition strategies in which novel probiotic formulations could be made, consisting of indigenous gut bacteria that will be effective on humans.
Data availability
Not applicable.
Code availability
Not applicable.
References
Afsahi A, Mahmoudi H, Ebrahimi A, Aeini Z, Esmaeili D. Evaluation of the effect of Lactobacillus planetarium probiotics produced from broad bean seed in prevention of Helicobacter pylori in stomach tissue of C57BL/6 mice. Journal of Cancer Science and Therapy. 10(4): 085-089 (2018)
Akbari V, Hendijani F. Effects of probiotic supplementation in patients with type 2 diabetes: systematic review and meta-analysis. Nutrition Reviews. 74(12): 774-784 (2016)
Al-Sheraji SH, Ismail A, Manap MY, Mustafa S, Yusof RM, Hassan FA. Prebiotics as functional foods: A review. Journal of Functional Foods. 5(4): 1542-1553 (2013).
Alissa EM, Ferns GA. Functional foods and nutraceuticals in the primary prevention of cardiovascular diseases. Journal of Nutrition Metabolism. 1: 569486 (2012)
Anderson JW, Gilliland SE. Effect of fermented milk (yogurt) containing Lactobacillus acidophilus L1 on serum cholesterol in hypercholesterolemic humans. Journal of the American College of Nutrition. 18(1): 43-50 (1999)
Anwer M, Siddique A, Ain N. Historical perspective of probiotics and role of regulating bodies globally. Food Science & Nutrition Technology. 4(6): 16000200 (2019)
Asemi Z, Zare Z, Shakeri H, Sabihi S-s, Esmaillzadeh A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Annals of Nutrition and Metabolism. 63(1-2): 1-9 (2013)
Ataie-Jafari A, Larijani B, Majd HA, Tahbaz F. Cholesterol-lowering effect of probiotic yogurt in comparison with ordinary yogurt in mildly to moderately hypercholesterolemic subjects. Annals of Nutrition and Metabolism. 54(1): 22-27 (2009)
Ballini A, Charitos IA, Cantore S, Topi S, Bottalico L, Santacroce L. About functional foods: The probiotics and prebiotics state of art. Antibiotics. 12(4): 635 (2023)
Barichella M, Pacchetti C, Bolliri C, Cassani E, Iorio L, Pusani C, Pinelli G, Privitera G, Cesari I, Faierman SA. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: an RCT. Neurology. 87(12): 1274-1280 (2016)
Barragán‐Martínez LP, Totosaus A, de Lourdes Pérez-Chabela M. Probiotication of cooked sausages employing agroindustrial coproducts as prebiotic co‐encapsulant in ionotropic alginate–pectin gels. International Journal of Food Science and Technology. 55(3): 1088-1096 (2020)
BBC. Probiotics in Food, Beverages, Dietary Supplements and Animal Feed. BBC Publishing (2020)
Boada CC, Martinez-Moreno JM. Pathophysiology of diabetes mellitus type 2: beyond the duo “insulin resistance-secretion deficit”. Nutricion Hospitalaria. 28(2): 78-87 (2013)
Burgain J, Gaiani C, Linder M, Scher J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications. Journal of Food Engineering. 104(4): 467-483 (2011)
Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 3(4): 279-288 (2012)
Chau K, Lau E, Greenberg S, Jacobson S, Yazdani-Brojeni P, Verma N, Koren G. Probiotics for infantile colic: a randomized, double-blind, placebo-controlled trial investigating Lactobacillus reuteri DSM 17938. The Journal of Pediatrics. 166(1): 74-78 e71 (2015)
Chen D, Yang Z, Chen X, Huang Y, Yin B, Guo F, Zhao H, Zhao T, Qu H, Huang J. The effect of Lactobacillus rhamnosus hsryfm 1301 on the intestinal microbiota of a hyperlipidemic rat model. BMC Complementary and Alternative Medicine. 14(1): 1-9 (2014)
Chen ME, Su CH, Yang JS, Lu CC, Hou YC, Wu JB, Hsu YM. Baicalin, baicalein, and Lactobacillus rhamnosus JB3 alleviated Helicobacter pylori infections in vitro and in vivo. Journal of Food Science. 83(12): 3118-3125 (2018)
Chen S-M, Chieng W-W, Huang S-W, Hsu L-J, Jan M-S. The synergistic tumor growth-inhibitory effect of probiotic Lactobacillus on transgenic mouse model of pancreatic cancer treated with gemcitabine. Scientific Reports. 10(1): 20319 (2020)
Chen Z, Liang W, Liang J, Dou J, Guo F, Zhang D, Xu Z, Wang T. Probiotics: functional food ingredients with the potential to reduce hypertension. Frontiers in Cellular and Infection Microbiology. 13: 1220877 (2023)
Chien YL, Wu LY, Lee TC, Hwang LS. Cholesterol-lowering effect of phytosterol-containing lactic-fermented milk powder in hamsters. Food Chemistry. 119(3): 1121-1126 (2010)
Chitapanarux I, Chitapanarux T, Traisathit P, Kudumpee S, Tharavichitkul E, Lorvidhaya V. Randomized controlled trial of live Lactobacillus acidophilus plus Bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiation Oncology. 5(1): 1-6 (2010)
de Almeida-Pititto B, Dias ML, de Moraes ACF, Ferreira SR, Franco DR, Eliaschewitz FG. Type 2 diabetes in Brazil: epidemiology and management. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 8: 17 (2015)
De Prisco A, Mauriello G. Probiotication of foods: A focus on microencapsulation tool. Trends in Food Science & Technology. 48: 27-39 (2016)
De Simone C. The unregulated probiotic market. Clinical Gastroenterology and Hepatology. 17(5): 809-817 (2019)
Dehghani N, Tafvizi F, Jafari P. Cell cycle arrest and anti-cancer potential of probiotic Lactobacillus rhamnosus against HT-29 cancer cells. BioImpacts: BI. 11(4): 245 (2021)
Delcenserie V, Martel D, Lamoureux M, Amiot J, Boutin Y, Roy D. Immunomodulatory effects of probiotics in the intestinal tract. Current Issues in Molecular Biology. 10(1-2): 37-54 (2008)
Duan H, Yu L, Tian F, Zhai Q, Fan L, Chen W. Gut microbiota: a target for heavy metal toxicity and a probiotic protective strategy. Science of The Total Environment. 742: 140429 (2020)
El-Nezami HS, Polychronaki NN, Ma J, Zhu H, Ling W, Salminen EK, Juvonen RO, Salminen SJ, Poussa T, Mykkänen HM. Probiotic supplementation reduces a biomarker for increased risk of liver cancer in young men from Southern China. The American Journal of Clinical Nutrition. 83(5): 1199-1203 (2006)
Elmståhl S, Svensson U, Berglund G. Fermented milk products are associated to ulcer disease. Results from a cross-sectional population study. European Journal of Clinical Nutrition. 52(9): 668-674 (1998)
Elshaghabee FM, Rokana N, Gulhane RD, Sharma C, Panwar H. Bacillus as potential probiotics: status, concerns, and future perspectives. Frontiers in Microbiology. 8: 1490 (2017)
Espitia PJ, Batista RA, Azeredo HM, Otoni CG. Probiotics and their potential applications in active edible films and coatings. Food Research International. 90: 42-52 (2016)
Evenepoel P, Poesen R, Meijers B. The gut–kidney axis. Pediatric Nephrology. 32(11): 2005-2014 (2017)
Flach J, van der Waal MB, van den Nieuwboer M, Claassen E, Larsen OF. The underexposed role of food matrices in probiotic products: Reviewing the relationship between carrier matrices and product parameters. Critical Reviews in Food Science Nutrition Research. 58(15): 2570-2584 (2018)
Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nature Reviews Gastroenterology and Hepatology. 9(10): 577-589 (2012)
Flora SJ, Pachauri V. Chelation in metal intoxication. International Journal of Environmental Research and Public Health. 7(7): 2745-2788 (2010)
Fuentes MC, Lajo T, Carrión JM, Cuné J. Cholesterol-lowering efficacy of Lactobacillus plantarum CECT 7527, 7528 and 7529 in hypercholesterolaemic adults. British Journal of Nutrition. 109(10): 1866-1872 (2013)
Fusco, V., Fanelli, F., & Chieffi, D. Authenticity of probiotic foods and dietary supplements: a pivotal issue to address. Critical Reviews in Food Science and Nutrition. 62(25): 6854–6871 (2022)
Gallaher DD, Stallings WH, Blessing LL, Busta FF, Brady LJ. Probiotics, cecal microflora, and aberrant crypts in the rat colon. The Journal of Nutrition. 126(5): 1362-1371 (1996)
Gerritsen J, Smidt H, Rijkers GT, de Vos WM, Nutrition. Intestinal microbiota in human health and disease: the impact of probiotics. Genes. 6(3): 209-240 (2011)
Ghoneum M, Felo N. Selective induction of apoptosis in human gastric cancer cells by Lactobacillus kefiri (PFT), a novel kefir product. Oncology Reports. 34(4): 1659-1666 (2015)
Gill HS, Guarner F. Probiotics and human health: a clinical perspective. Postgraduate Medical Journal. 80(947): 516-526 (2004)
Gorbach SL, Newton I. The discovery of Lactobacillus GG. Nutrition Today. 31(6): 5S (1996)
Guandalini S. Probiotics for prevention and treatment of diarrhea. Journal of Clinical Gastroenterology. 45: S149-S153 (2011)
Gui Q, Lu H, Zhang C, Xu Z, Yang Y. Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genetics and Molecular Research. 14(2): 5642-5651 (2015)
Guida B, Germanò R, Trio R, Russo D, Memoli B, Grumetto L, Barbato F, Cataldi M. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: a randomized clinical trial. Nutrition, Metabolism and Cardiovascular Diseases. 24(9): 1043-1049 (2014)
Guo Z, Liu X, Zhang Q, Shen Z, Tian F, Zhang H, Sun Z, Zhang H, Chen W. Influence of consumption of probiotics on the plasma lipid profile: a meta-analysis of randomised controlled trials. Nutrition, Metabolism and Cardiovascular Diseases. 21(11): 844-850 (2011)
Hasler CM. Functional foods: benefits, concerns and challenges—a position paper from the American Council on Science and Health. The Journal of Nutrition. 132(12): 3772-3781 (2002)
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology and Hepatology. 11(8): 506-514 (2014)
Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. New England Journal of Medicine. 345(11): 790-797 (2001)
Inbaraj BS, Chen B-H. In vitro removal of toxic heavy metals by poly (γ-glutamic acid)-coated superparamagnetic nanoparticles. International Journal of Nanomedicine. 7: 4419 (2012)
Intelligence M. Global probiotics market: Growth, trends, and forecast (2020-2025). Available from https://www.mordorintelligence.com/industry-reports/probiotics-market (2019)
Irecta-Nájera CA, del Rosario Huizar-López M, Casas-Solís J, Castro-Félix P, Santerre A. Protective effect of Lactobacillus casei on DMH-induced colon carcinogenesis in mice. Probiotics and Antimicrobial Proteins. 9(2): 163-171 (2017)
Jacouton E, Chain F, Sokol H, Langella P, Bermudez-Humaran LG. Probiotic strain Lactobacillus casei BL23 prevents colitis-associated colorectal cancer. Frontiers in Immunology. 8: 1553 (2017)
Jaskulski IB, Uecker J, Bordini F, Moura F, Gonçalves T, Chaves NG, Camargo F, Grecco FB, Fiorentini ÂM, da Silva WP. In vivo action of Lactococcus lactis subsp. lactis isolate (R7) with probiotic potential in the stabilization of cancer cells in the colorectal epithelium. Process Biochemistry. 91: 165-171 (2020)
Jeon SG, Kayama H, Ueda Y, Takahashi T, Asahara T, Tsuji H, Tsuji NM, Kiyono H, Ma JS, Kusu T. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathogens. 8(5): e1002714 (2012)
Kaur S, Das M. Functional foods: An overview. Food Science and Biotechnology. 20: 861-875 (2011)
Keikha M, Karbalaei M. Probiotics as the live microscopic fighters against Helicobacter pylori gastric infections. BMC Gastroenterology. 21(1): 1-18 (2021)
Khoury T, Tzukert K, Abel R, Abu Rmeileh A, Levi R, Ilan Y. The gut‐kidney axis in chronic renal failure: A new potential target for therapy. Hemodialysis International. 21(3): 323-334 (2017)
Kim B, Park K-Y, Ji Y, Park S, Holzapfel W, Hyun C-K. Protective effects of Lactobacillus rhamnosus GG against dyslipidemia in high-fat diet-induced obese mice. Biochemical and Biophysical Research Communications. 473(2): 530-536 (2016)
Kim S-J, Park SH, Sin H-S, Jang S-H, Lee S-W, Kim S-Y, Kwon B, Yu K-Y, Kim SY, Yang DK. Hypocholesterolemic effects of probiotic mixture on diet-induced hypercholesterolemic rats. Nutrients. 9(3): 293 (2017)
Koppe L, Mafra D, Fouque D. Probiotics and chronic kidney disease. Kidney International. 88(5): 958-966 (2015)
Kotilainen, Liisa, Riikka Rajalahti, Catherine Ragasa, and Eija Pehu. Health enhancing foods: opportunities for strengthening developing countries, 1 (2006)
Kumar M, Nagpal R, Kumar R, Hemalatha R, Verma V, Kumar A, Chakraborty C, Singh B, Marotta F, Jain S. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Journal of Diabetes Research. 1, 902917 (2012)
Kwak H-S. Nano-and Microencapsulation for Foods. John Wiley & Sons (2014)
Li X, Xu Q, Jiang T, Fang S, Wang G, Zhao J, Zhang H, Chen W. A comparative study of the antidiabetic effects exerted by live and dead multi-strain probiotics in the type 2 diabetes model of mice. Food and Function. 7(12): 4851-4860 (2016)
Lin C-C, Huang W-C, Su C-H, Lin W-D, Wu W-T, Yu B, Hsu Y-M. Effects of multi-strain probiotics on immune responses and metabolic balance in Helicobacter pylori-infected mice. Nutrients. 12(8): 2476 (2020)
Liu Y, Tran DQ, Rhoads JM. Probiotics in disease prevention and treatment. The Journal of Clinical Pharmacology. 58: S164-S179 (2018)
Liu Z, Qin H, Yang Z, Xia Y, Liu W, Yang J, Jiang Y, Zhang H, Yang Z, Wang Y. Randomised clinical trial: the effects of perioperative probiotic treatment on barrier function and post‐operative infectious complications in colorectal cancer surgery–a double‐blind study. Alimentary Pharmacology & Therapeutics. 33(1): 50-63 (2011)
Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy Reviews. 4(8): 118 (2010)
Longenecker JC, Coresh J, Powe NR, Levey AS, Fink NE, Martin A, Klag MJ. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE Study. Journal of the American Society of Nephrology. 13(7): 1918-1927 (2002)
Ly LD, Xu S, Choi S-K, Ha C-M, Thoudam T, Cha S-K, Wiederkehr A, Wollheim CB, Lee I-K, Park K-S. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Experimental and Molecular Medicine. 49(2): e291 (2017)
Lykova E, Bondarenko V, Sidorenko S, Grishina M, Murashova A, Minaev V, Rytikov F, Korsunskiĭ A. Combined antibacterial and probiotic therapy of Helicobacter-associated diseases in children. Zhurnal Mikrobiologii, Epidemiologii i Immunobiologii (2): 76-81 (1999)
Majamaa H, Isolauri E. Probiotics: a novel approach in the management of food allergy. Journal of Allergy and Clinical Immunology. 99(2): 179-185 (1997)
Memarrast F, Ghafouri‐Fard S, Kolivand S, Jafary‐Nodooshan S, Neyazi N, Sadroddiny E, Motevaseli E. Comparative evaluation of probiotics effects on plasma glucose, lipid, and insulin levels in streptozotocin‐induced diabetic rats. Diabetes/Metabolism Research and Reviews. 33(7): e2912 (2017)
Menrad K. Market and marketing of functional food in Europe. Journal of Food Engineering. 56(2-3): 181-188 (2003)
Merino J, García A, Pastene E, Salas A, Saez K, González C. Lactobacillus fermentum UCO-979C strongly inhibited Helicobacter pylori SS1 in Meriones unguiculatus. Beneficial Microbes. 9(4): 625-627 (2018)
Miraghajani M, Zaghian N, Mirlohi M, Feizi A, Ghiasvand R. The impact of probiotic soy milk consumption on oxidative stress among type 2 diabetic kidney disease patients: a randomized controlled clinical trial. Journal of Renal Nutrition. 27(5): 317-324 (2017)
Misra S, Pandey P, Mishra HN, Technology. Novel approaches for co-encapsulation of probiotic bacteria with bioactive compounds, their health benefits and functional food product development: A review. Trends in Food Science. 109: 340-351 (2021)
Navab-Moghadam F, Sedighi M, Khamseh ME, Alaei-Shahmiri F, Talebi M, Razavi S, Amirmozafari N. The association of type II diabetes with gut microbiota composition. Microbial Pathogenesis. 110: 630-636 (2017)
Neto MPC, de Souza Aquino J, da Silva LdFR, de Oliveira Silva R, de Lima Guimaraes KS, de Oliveira Y, de Souza EL, Magnani M, Vidal H, de Brito Alves JL. Gut microbiota and probiotics intervention: a potential therapeutic target for management of cardiometabolic disorders and chronic kidney disease? Pharmacological Research. 130: 152–163 (2018)
Ohigashi S, Hoshino Y, Ohde S, Onodera H. Functional outcome, quality of life, and efficacy of probiotics in postoperative patients with colorectal cancer. Surgery Today. 41: 1200-1206 (2011)
Organisation WWG. Global Guidelines: Probiotics and Prebiotics (2017)
Palanivelu J, Thanigaivel S, Vickram S, Dey N, Mihaylova D, Desseva I. Probiotics in functional foods: Survival assessment and approaches for improved viability. Applied Sciences. 12(1): 455 (2022)
Patel A, Shah N, Prajapati J. Clinical application of probiotics in the treatment of Helicobacter pylori infection—a brief review. Journal of Microbiology, Immunology and Infection. 47(5): 429-437 (2014)
Pessi T, Sütas Y, Marttinen A, Isolauri E. Probiotics reinforce mucosal degradation of antigens in rats: implications for therapeutic use of probiotics. The Journal of Nutrition. 128(12): 2313-2318 (1998)
Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Advances in Nutrition. 10(suppl_1): S49-S66 (2019)
Poesen R, Meijers B, Evenepoel P. The colon: an overlooked site for therapeutics in dialysis patients. Seminars in Dialysis 26(3): 323–332 (2013)
Poli A, Barbagallo CM, Cicero AF, Corsini A, Manzato E, Trimarco B, Bernini F, Visioli F, Bianchi A, Canzone G. Nutraceuticals and functional foods for the control of plasma cholesterol levels. An intersociety position paper. Pharmacological Research. 134: 51-60 (2018)
Pourbaferani M, Modiri S, Norouzy A, Maleki H, Heidari M, Alidoust L, Derakhshan V, Zahiri HS, Noghabi KA. A newly characterized potentially probiotic strain, Lactobacillus brevis MK05, and the toxicity effects of its secretory proteins against MCF-7 breast cancer cells. Probiotics and Antimicrobial Proteins. 13: 982-992 (2021)
Pourmasoumi M, Najafgholizadeh A, Hadi A, Mansour-Ghanaei F, Joukar F. The effect of synbiotics in improving Helicobacter pylori eradication: A systematic review and meta-analysis. Complementary Therapies in Medicine. 43: 36-43 (2019)
Prakash S, Chang T. Microencapsulated genetically engineered live E. coli DH5 cells administered orally to maintain normal plasma urea level in uremic rats. Nature Medicine. 2(8): 883-887 (1996)
Rafter J, Bennett M, Caderni G, Clune Y, Hughes R, Karlsson PC, Klinder A, O’Riordan M, O’Sullivan GC, Pool-Zobel B. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. The American Journal of Clinical Nutrition. 85(2): 488-496 (2007)
Ranganathan N, Patel B, Ranganathan P, Marczely J, Dheer R, Chordia T, Dunn SR, Friedman EA. Probiotic amelioration of azotemia in 5/6th nephrectomized Sprague-Dawley rats. The Scientific World Journal. 5: 652-660 (2005)
Ranganathan N, Ranganathan P, Friedman EA, Joseph A, Delano B, Goldfarb DS, Tam P, Venketeshwer Rao A, Anteyi E, Guido Musso C. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Advances in Therapy. 27(9): 634-647 (2010)
Rani, B, Khetarpaul, N. Probiotic fermented food mixtures: possible applications in clinical anti-diarrhoea usage. Nutrition and Health. 12(2): 97-105 (1998)
Rasouli BS, Ghadimi-Darsajini A, Nekouian R, Iragian G-R. In vitro activity of probiotic Lactobacillus reuteri against gastric cancer progression by downregulation of urokinase plasminogen activator/urokinase plasminogen activator receptor gene expression. Journal of Cancer Research and Therapeutics. 13(2): 246-251 (2017)
Reque PM, Brandelli A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends in Food Science and Technology. 114: 1-10 (2021)
Roberfroid M, Bornet F, Bouley Ce, Cummings J. Colonic microflora: nutrition and health. Summary and conclusions of an International Life Sciences Institute (ILSI) [Europe] workshop held in Barcelona, Spain. Nutrition Reviews. 53(5): 127-130 (1995)
Rudinsky AJ, Harrison A, Shi B, Hardison R, Prinster T, Huang S, Lee S, Byron JK, Lucas E, Mason KM. The use of Escherichia coli strain Nissle 1917 shows promise for improving gastrointestinal and urinary health in dogs. American Journal of Veterinary Research. 84(8): 0055 (2023)
Saavedra-Leos MZ, Román-Aguirre M, Toxqui-Terán A, Espinosa-Solís V, Franco-Vega A, Leyva-Porras C. Blends of carbohydrate polymers for the co-microencapsulation of Bacillus clausii and quercetin as active ingredients of a functional food. Polymers. 14(2): 236 (2022)
Sarao LK, Arora M. Probiotics, prebiotics, and microencapsulation: A review. Critical Reviews in Food Science and Nutrition. 57(2): 344-371 (2017)
Shadnoush M, Hosseini RS, Khalilnezhad A, Navai L, Goudarzi H, Vaezjalali M. Effects of probiotics on gut microbiota in patients with inflammatory bowel disease: a double-blind, placebo-controlled clinical trial. The Korean Journal of Gastroenterology. 65(4): 215-221 (2015)
Shi X, Zhang J, Mo L, Shi J, Qin M, Huang X. Efficacy and safety of probiotics in eradicating Helicobacter pylori: A network meta-analysis. Medicine. 98(15): p e15180 (2019)
Shimizu M., Hashiguchi M, Shiga T, Tamura H-o, Mochizuki M. Meta-analysis: effects of probiotic supplementation on lipid profiles in normal to mildly hypercholesterolemic individuals. PLoS ONE. 10(10): e0139795 (2015)
Simenhoff M, Dunn S, Zollner G, Fitzpatrick M, Emery S, Sandine W, Ayres J. Biomodulation of the toxic and nutritional effects of small bowel bacterial overgrowth in end-stage kidney disease using freeze-dried Lactobacillus acidophilus. Mineral and Electrolyte Metabolism. 22(1-3): 92-96 (1996)
Siró I, Kápolna E, Kápolna B, Lugasi A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite. 51(3): 456-467 (2008)
Sloan AE. Top ten functional food trends. Food Technology. 10 (2000)
Sloan AE. The top 10 functional food trends: the next generation. Food Technology. 56: 32-57 (2002)
Sloan AE. The top 10 functional food trends. Food Technology. 58(4): 28-51 (2004)
Soleimani A, Mojarrad MZ, Bahmani F, Taghizadeh M, Ramezani M, Tajabadi-Ebrahimi M, Jafari P, Esmaillzadeh A, Asemi Z. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney International. 91(2): 435-442 (2017)
Song E-J, Han K, Lim T-J, Lim S, Chung M-J, Nam MH, Kim H, Nam Y-D. Effect of probiotics on obesity-related markers per enterotype: a double-blind, placebo-controlled, randomized clinical trial. EPMA Journal. 11(1): 31-51 (2020)
Song M, Park S, Lee H, Min B, Jung S, Kim E, Oh S. Effect of Lactobacillus acidophilus NS1 on plasma cholesterol levels in diet-induced obese mice. Journal of Dairy Science. 98(3): 1492-1501 (2015)
Télessy, István G. “Nutraceuticals.” The role of functional food security in global health. Academic Press, 409–421 (2019)
Terpou A, Papadaki A, Lappa IK, Kachrimanidou V, Bosnea LA, Kopsahelis N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients. 11(7): 1591 (2019)
Uccello M, Malaguarnera, G, Basile F, D’agata V, Malaguarnera M, Bertino G, Vacante M, Drago F, Biondi A. Potential role of probiotics on colorectal cancer prevention. BMC surgery, 12(1): 1-8 (2012)
Valdemiro Carlos, S. The importance of prebiotics in functional foods and clinical practice. Food and Nutrition Sciences 2(2): 133–144 (2011)
Vaziri, N. D. CKD impairs barrier function and alters microbial flora of the intestine: a major link to inflammation and uremic toxicity. Current opinion in nephrology and hypertension, 21(6): 587 (2012)
Vergari F, Tibuzzi A, Basile G. An overview of the functional food market: from marketing issues and commercial players to future demand from life in space. pp. 308–321. In: Bio-farms for Nutraceuticals: Functional Food and Safety Control by Biosensors (2010)
Vicas SI, Laslo V, Timar AV, Balta C, Herman H, Ciceu A, Gharbia S, Rosu M, Mladin B, Chiana L. Nano selenium—Enriched probiotics as functional food products against cadmium liver toxicity. Materials. 14(9): 2257 (2021)
Wang G, Li X, Zhao J, Zhang H, Chen W. Lactobacillus casei CCFM419 attenuates type 2 diabetes via a gut microbiota dependent mechanism. Food and Function. 8(9): 3155-3164 (2017)
Wang X, Juan Q-F, He Y-W, Zhuang L, Fang Y-Y, Wang Y-H. Multiple effects of probiotics on different types of diabetes: a systematic review and meta-analysis of randomized, placebo-controlled trials. Journal of Pediatric Endocrinology and Metabolism. 30(6): 611-622 (2017)
Xiao Y, Lu C, Liu Y, Kong L, Bai H, Mu H, Li Z, Geng H, Duan J. Encapsulation of Lactobacillus rhamnosus in hyaluronic acid-based hydrogel for pathogen-targeted delivery to ameliorate enteritis. ACS Applied Materials & Interfaces. 12(33): 36967-36977 (2020)
Yang J, Hou B, Wang J, Tian B, Bi J, Wang N, Li X, Huang X. Nanomaterials for the removal of heavy metals from wastewater. Nanomaterials. 9(3): 424 (2019)
Yaqoob P. Ageing, immunity and influenza: a role for probiotics? Proceedings of the Nutrition Society. 73(2): 309-317 (2014)
Yoon H, Park YS, Lee DH, Seo J-G, Shin CM, Kim NJ. Effect of administering a multi-species probiotic mixture on the changes in fecal microbiota and symptoms of irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. Journal of Clinical Biochemistry Nutrition Research. 57(2): 129–134 (2015)
Yu M, Zhang R, Ni P, Chen S, Duan G. Efficacy of Lactobacillus-supplemented triple therapy for H. pylori eradication: A meta-analysis of randomized controlled trials. PLoS ONE. 14(10): e0223309 (2019)
Yue Y, Ye K, Lu J, Wang X, Zhang S, Liu L, Yang B, Nassar K, Xu X, Pang X. Probiotic strain Lactobacillus plantarum YYC-3 prevents colon cancer in mice by regulating the tumour microenvironment. Biomedicine & Pharmacotherapy. 127: 110159 (2020)
Zamani B, Golkar HR, Farshbaf S, Emadi‐Baygi M, Tajabadi‐Ebrahimi M, Jafari P, Akhavan R, Taghizadeh M, Memarzadeh MR, Asemi Z. Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: a randomized, double‐blind, placebo‐controlled trial. International Journal of Rheumatic Diseases. 19(9): 869-879 (2016)
Zheng C, Chen T, Lu J, Wei K, Tian H, Liu W, Xu T, Wang X, Wang S, Yang R. Adjuvant treatment and molecular mechanism of probiotic compounds in patients with gastric cancer after gastrectomy. Food & Function. 12(14): 6294-6308 (2021)
Zhong L, Zhang X, Covasa M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World Journal of Gastroenterology: WJG. 20(24): 7878 (2014)
Zhou BG, Chen LX, Li B, Wan LY, Ai YW. Saccharomyces boulardii as an adjuvant therapy for Helicobacter pylori eradication: A systematic review and meta‐analysis with trial sequential analysis. Helicobacter. 24(5): e12651 (2019)
Zubillaga M, Weill R, Postaire E, Goldman C, Caro R, Boccio J. Effect of probiotics and functional foods and their use in different diseases. Nutrition Research. 21(3): 569-579 (2001)
Zheng J, Wittouck S, Salvetti E, Franz CM, Harris HM, Mattarelli P, O’toole PW, Pot B, Vandamme P, Walter J, Watanabe K, Wuyts S, Felis GE, Gänzle MG, Lebeer S. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. International Journal of Systematic and Evolutionary Microbiology. 70(4): 2782-2858 (2020)
Acknowledgements
The authors have no acknowledgments to declare.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors of this study has any financial interest or conflict with industries or parties.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anwer, M., Wei, M.Q. Harnessing the power of probiotic strains in functional foods: nutritive, therapeutic, and next-generation challenges. Food Sci Biotechnol (2024). https://doi.org/10.1007/s10068-024-01630-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10068-024-01630-z