Abstract
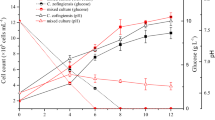
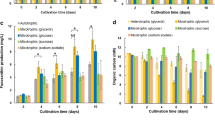
This work investigates bacterial production of astaxanthin as an alternative to production by algae and yeast owing to its lower incubation time and simpler downstream processing. The physical parameters and medium composition were optimized for astaxanthin production by Paracoccus MBIC 01143. The optimized media was supplemented with tricarboxylic acid intermediates to enhance the pool of precursors, while the cofactors of Crt enzymes (ferrous sulphate, ascorbate, NADPH, ATP, and 2-oxoglutarate) were added to stimulate their enzyme activity aiming at higher astaxanthin accumulation. Malate at 5 mM and ferrous sulphate at 1 mM increased the astaxanthin production from 177 to 3,750 μg/L.
Similar content being viewed by others
References
Sandmann G, Albrecht M, Schnurr G, Knörzer O, Börer P. The biotechnological potential and design of novel carotenoids by gene combination in Escherichia coli. Trends Biotechnol. 17: 233–237 (1999
Bhosale P, Bernstein PS. Microbial xanthophylls. Appl. Microbiol. Biot. 68: 445–455 (2005)
Kamata T, Simpson KL. Study of astaxanthin diester extracted from Adonis aestivalis. Comp. Biochem. Phys. B 86: 587–591 (1987)
Ruen-ngam D, Shotipruk A, Pavasant P. Comparison of extraction methods for recovery of astaxanthin from Haematococcus pluvialis. Separ. Sci. Technol. 46: 64–70 (2011)
Yokoyama A, Izumida H, Miki W. Production of astaxanthin and 4-ketozeaxanthin by the marine bacterium, Agrobacterium aurantiacum. Biosci. Biotech. Bioch. 58: 1842–1844 (1994)
Harker M, Hirschberg J, Oren A. Paracoccus marcusii sp. nov., an orange Gram-negative coccus. Int. J. Syst. Bacteriol. 48: 543–548 (1998)
Tsubokura A, Yoneda H, Mizuta H. Paracoccus carotinifaciens sp. nov., a new aerobic Gram-negative astaxanthin-producing bacterium. Int. J. Syst. Bacteriol. 49: 277–282 (1999)
Lee JH, Kim YS, Choi TJ, Lee WJ, Kim YT. Paracoccus haeundaensis sp. nov., a Gram-negative, halophilic, astaxanthinproducing bacterium. Int. J. Syst. Evol. Micr. 4: 1699–1702 (2004)
Asker D, Beppu T, Ueda K. Sphingomonas astaxanthinifaciens sp. nov., a novel astaxanthin-producing bacterium of the family Sphingomonadaceae isolated from Misasa, Tottori, Japan. FEMS Microbiol. Lett. 273: 140–148 (2007)
Misawa N, Satomi Y, Kondo K, Yokoyama A, Kajiwara S, Saito T, Ohtani T, Miki W. Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. J. Bacteriol. 11: 6575–6584 (1995)
Fraser PD, Miura Y, Misawa N. In vitro characterization of astaxanthin biosynthetic enzymes. J. Biol. Chem. 272: 6128–6135 (1997)
MacLeo RA, Onofrey E, Norris M. Nutrition and metabolism of marine bacteria. I. Survey of nutritional requirements. J. Bacteriol. 68: 680–686 (1954)
Bhosale P, Gadre RV. Production of β-carotene by a Rhodotorula glutinis mutant in seawater medium. Bioresource Technol. 76: 53–55 (2001)
Durmaz Y, Donato M, Monteiro M, Gouveia L, Nunes ML, Gama Pereira T, Gökpinar Ş, Bandarra NM. Effect of temperature on α-tocopherol, fatty acid profile, and pigments of Diacronema vlkianum (Haptophyceae). Aquacult. Int. 17: 391–399 (2009)
Bhosale P, Gadre RV. Manipulation of temperature and illumination conditions for enhanced β-carotene production by mutant 32 of Rhodotorula glutinis. Lett. Appl. Microbiol. 34: 349–353 (2002)
Bhosale P. Environmental and cultural stimulants in the production of carotenoids from microorganisms. Appl. Microbiol. Biot. 63: 351–361 (2004)
Zhang DH, Lee YK, Ng ML, Phang SM. Composition and accumulation of secondary carotenoids in Chlorococcum sp. J. Appl. Phycol. 9: 147–155 (1997)
Liu Y-S, Wu J-Y, Ho K-P. Characterization of oxygen transfer conditions and their effects on Phaffia rhodozyma growth and carotenoid production in shake-flask cultures. Biochem. Eng. J. 27: 331–335 (2006)
Fakas S, Makri A, Bellou S, Aggelis G. Pathways to aerobic glycerol catabolism and their regulation. pp. 9–18. In: Microbial Conversions of Raw Glycerol. Aggelis G (ed). Nova Science Publishers, New York, NY, USA (2009)
Kusdiyantini E, Gaudin P, Goma G, Blanc PJ. Growth kinetics and astaxanthin production of Phaffia rhodozyma on glycerol as a carbon source during batch fermentation. Biotechnol. Lett. 20: 929–934 (1998)
Masojídek J, Torzillo G, Kopecky J, Koblžek M, Nidiaci L, Komenda J, Lukavská A, Sacchi, A. Changes in chlorophyll fluorescence quenching and pigment composition in the green alga Chlorococcum sp. grown under nitrogen deficiency and salinity stress. J. Appl. Phycol. 12: 417–426 (2000)
Withers ST, Keasling JD. Biosynthesis and engineering of isoprenoid small molecules. Appl. Microbiol. Biot. 73: 980–990 (2007)
Bhosale P, Larson J, Bernstein S. Factorial analysis of tricarboxylic acid cycle intermediates for optimization of zeaxanthin production from Flavobacterium multivorum. J. Appl. Microbiol. 96: 623–629 (2004)
Nasri Nasrabadi MR, Razavi SH. Use of response surface methodology in a fed-batch process for optimization of tricarboxylic acid cycle intermediates to achieve high levels of canthaxanthin from Dietzia natronolimnaea HS-1. J. Biosci. Bioeng. 109: 361–368 (2010)
Alcantara S, Sanchez S. Influence of carbon and nitrogen sources on Flavobacterium growth and zeaxanthin biosynthesis. J. Ind. Microbiol. Biot. 23: 697–700 (1999)
An G-H. Improved growth of the red yeast, Phaffia rhodozyma (Xanthophyllomyces dendrorhous), in the presence of tricarboxylic acid cycle intermediates. Biotechnol. Lett. 23: 1005–1009 (2001)
Domínguez-Bocanegra AR, Ponce-Noyola T, Torres-Muñoz JA. Astaxanthin production by Phaffia rhodozyma and Haematococcus pluvialis: A comparative study. Appl. Microbiol. Biot. 75: 783–791 (2007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chougle, J.A., Singhal, R.S. Metabolic precursors and cofactors stimulate astaxanthin production in Paracoccus MBIC 01143. Food Sci Biotechnol 21, 1695–1700 (2012). https://doi.org/10.1007/s10068-012-0225-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-012-0225-8