Abstract
We aimed to clarify the long-term safety and efficacy of rituximab (RTX) as a remission induction therapy following severe relapse in patients with antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV). We retrospectively collected the data of patients with severely relapsed AAV from a Japanese multicentre cohort. The primary exposure was RTX use; the primary outcome was complete remission (CR) proportions at week 24. Baseline characteristics were compared between the RTX and non-RTX groups. We performed multivariate logistic regression analysis and one-to-one propensity score matching analysis as a sensitivity analysis. Totally, 100 patients were enrolled: 52 in the RTX group and 48 in the non-RTX group. Baseline characteristics were comparable between the two groups, except for age, AAV subtype and ANCA serotype. The median age was 71 vs. 75 years, and the PR3-ANCA positivity rate was 44.2% vs. 18.8% in the RTX and non-RTX groups, respectively. No significant difference was observed in CR proportions at week 24 between the two groups (79.2% vs. 68.1%, p = 0.321), with an adjusted odds ratio of 1.27 (95% confidence interval [CI] 0.47–3.51). At week 48, CR proportions were significantly higher in the RTX group (91.7% vs. 64.9%, p = 0.005), with an adjusted odds ratio of 2.95 (95% CI 0.97–9.91). Serious infection rates were lower in the RTX group than in the non-RTX group, with no statistically significant difference. RTX was not superior to conventional immunosuppressive therapies at week 24 but showed significantly favourable results at week 48 for severely relapsed AAV.
Key Points |
• RTX might be superior to traditional treatments as an induction therapy for severely relapsed AAV. • RTX has the potential to sustain long-term remission with fewer occurrences of infections in the treatment of severely relapsed AAV. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV) is a severe systemic small-vessel disease characterized by the presence of autoantibodies such as autoantibodies against neutrophil proteins, leukocyte proteinase 3 (PR3-ANCA) and myeloperoxidase (MPO-ANCA) and categorized into granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA) and eosinophilic GPA (EGPA) based on clinical features [1].
Recently, early diagnosis followed by prompt treatment improved the 5-year survival rate of patients with AAV. However, high relapse rates after remission remain a concern. Depending on the treatment, relapse rates of 21–89% have been reported to occur 5 years after the initiation of induction therapies [2]. Another review reported that one-third of the patients with recurrence had a severe disease [1].
Historically, cyclophosphamide has been commonly used in remission induction therapy for severe and relapsed cases. However, the side effects associated with cumulative doses of cyclophosphamide have become problematic, leading to a shift toward rituximab (RTX) as the primary therapy. RTX, an anti-CD20 monoclonal antibody, plays a key role in AAV treatment by depleting B cells [3,4,5]. RTX has been shown to be effective in treating AAV, with remission rates comparable to that of cyclophosphamide [6]. In addition, RTX has demonstrated a tolerable safety profile across diverse age groups [7,8,9]. Recent studies have reported promising results regarding the effectiveness of RTX in relapsed AAV [10,11,12].
However, recent studies assessing the effects of RTX on recurrent AAV, which included patients from the RITAZAREM and RAVE trials, wherein some relapses were mild, have predominantly focused on short-term outcomes [10, 11]. Another recent study from Japan compared the effectiveness and safety of RTX and intravenous cyclophosphamide for the treatment of life-threatening AAV [13]. However, the limitations of this study are that it focused on short-term outcomes over a 60-day period and did not distinguish between new-onset and recurrent cases. Moreover, because it was based on data extracted from a nationwide inpatient database in Japan, detailed information on disease activity, diagnostic validity, laboratory data and therapeutic agents was unavailable. Therefore, a more comprehensive exploration of RTX use in severe relapse scenarios is required. Current studies leave a significant gap in the understanding of the long-term effectiveness and safety of RTX as a remission induction therapy after severe relapse in real-world settings.
Therefore, we examined the effectiveness of RTX in treating patients with relapsed AAV in Japan, using multicentre registry data. This study is pivotal for improving the care of patients with AAV in Japan and other regions.
Materials and methods
Study design
We conducted a multicentre retrospective cohort analysis using data from the Japan Collaborative Registry of ANCA-Associated Vasculitis (J-CANVAS), a multicentre registry established by 24 referral sites in Japan.
Setting
The registry enrolled adult patients (aged ≥ 20 years) who were newly diagnosed with AAV or experienced a relapse between January 2017 and June 2020. All patients were classified as having MPA, GPA or EGPA based on the definitions of the 2012 International Chapel Hill Consensus Conference and European Medicines Agency algorithm [14]. The duration of follow-up for each patient ranged from the onset of disease to the occurrence of mortality, loss to follow-up or June 2021.
Participants
This study included patients with MPA and GPA who had achieved remission (Birmingham Vasculitis Activity Score [BVAS] = 0) and subsequently experienced severe relapses, defined as life- or organ-threatening, to determine the effectiveness of RTX [15].
Data collection
We retrospectively collected clinical information from the clinical records of each medical site. Baseline characteristics were collected before initiating or enhancing treatment, including patient demographics (age, sex, AAV subtypes [MPA/GPA/EGPA] and ANCA serotypes [MPO-ANCA/PR3-ANCA]). Additionally, data on various factors were collected, including the BVAS 3.0 at relapse [16], medication received at relapse (such as glucocorticoids and immunosuppressants), achievement of complete remission (CR) induction at 24 and 48 weeks after relapse, treatment with concomitant immunosuppressants during the follow-up period and the incidence of severe infections.
Exposures
Primary exposure was defined as the administration of RTX at least once after relapse, categorizing the administration frequency as one, two, three or four times. The dose and frequency of RTX were determined by each clinician.
Outcomes
The primary outcome was the proportion of patients achieving CR at 24 weeks. CR was defined as a BVAS of 0, irrespective of the use of immunosuppressive drugs. Secondary outcomes were the proportion of patients with CR at 48 weeks, BVAS at 24 and 48 weeks, severe infections occurring within the 48-week period and glucocorticoid dosage during the follow-up period.
Statistical analysis
Age, glucocorticoid dosage and BVAS were the continuous variables. Sex, ANCA serotype, AAV subtype, concurrent medication, achievement of CR and occurrence of serious infection were the categorical variables. Summary statistics were presented as median values and quartile ranges or as numbers with proportions. We conducted univariate analysis of the variables between the RTX and non-RTX groups. Continuous variables were analysed using the Wilcoxon rank sum test, and categorical variables were analysed using the chi-square test.
We conducted logistic multivariate analysis to estimate the odds ratio of achieving CR with RTX use. We selected sex, AAV subtype, ANCA serotype and glucocorticoid dosage at reinduction treatment as covariates because of their clinical relevance in achieving CR in patients with AAV, as previously reported [17, 18]. We conducted subgroup analyses to explore the interactions of RTX with ANCA serotype and AAV subtype.
We assumed that collected data were randomly missing. Consequently, multiple imputations were performed for outcomes used in multivariate analysis, and 50 imputed datasets were created. We defined cases of death at 24 and 48 weeks as non-remission.
In the sensitivity analysis, we performed a propensity score matching analysis between the RTX and non-RTX groups. We used a logistic regression model to estimate the propensity scores and applied the nearest neighbour matching with a calliper of 0.25. The matching ratio was 1:1 based on age, AAV subtype, ANCA serotype, initial glucocorticoid dosage and initial BVAS score at relapse. Additionally, we conducted a complete case analysis. Finally, we confined the control group to a non-RTX cohort treated with cyclophosphamide, assuming equivalent therapeutic effects to RTX.
Statistical significance was defined as a two-sided p-value < 0.05. All statistical analyses were performed using the R software (version 4.2.2).
Results
Study population and background characteristics
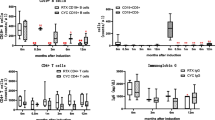
One hundred patients were included in the analysis; Table 1 shows the baseline characteristics of the participants. Details of organ involvement are provided in Supplementary Table 1. The RTX group had a higher prevalence of GPA and PR3-ANCA. Furthermore, the BVAS at relapse was not significantly different between the two groups (9 vs. 10 points). The dosage of glucocorticoids (prednisolone equivalent) administered was similar in both groups (Fig. 1). For induction therapy, RTX was administered at a standard dose of 375 mg/m2, with patients receiving between one and four doses: one dose in five patients, two in six, three in seven and four in 34. RTX for maintenance was administered to 30 of 52 patients 6 months after induction at doses of 500 mg/body or 375 mg/m2.
CR at week 24
The proportion of patients who achieved CR at week 24 was higher in the RTX group than in the non-RTX group; however, the difference was not statistically significant (38 [79.2%] vs. 32 [68.1%], p = 0.321) (Fig. 2; Table 2). The adjusted odds ratios for achieving CR at 24 weeks with the use of RTX after multiple imputations were 1.27 (95% confidence interval [CI] 0.47–3.51) (Table 3).
Sankey diagram illustrating proportions of complete remission, non-complete remission, missing/withdrawal and death at baseline, 24 weeks and 48 weeks in the RTX (left) and non-RTX groups (right). Patients who achieved CR are classified as “complete remission”, those who did not achieve CR as “non-remission”, those with missing data or who withdrew from the registry as “missing/withdrawn” and those who passed away as “death”. The values in the figure represent the number of individuals and their proportions at each time point. RTX, rituximab; CR, complete remission
CR at week 48 and the incidence of severe infection
At week 48, the RTX group had a significantly higher proportion of patients who achieved CR (p = 0.005) (Table 2). The adjusted odds ratio for achieving CR at 48 weeks with the use of RTX after multiple imputations was 2.95 (95% CI 0.97–9.91) (Table 3). Regarding safety, the incidence of severe infections tended to be lower in the RTX group than in the non-RTX group [8 (15.4%) vs. 12 (25.0%), p = 0.342] (Table 2).
Subgroup analyses by ANCA serotype and AAV subtype
Subgroup analyses to assess the interactions of RTX with ANCA serotype and AAV subtype showed no apparent qualitative interaction in RTX effect (Fig. 3).
Subgroup analysis by ANCA serotype and AAV subtype on the association between RTX use and complete remission. The forest plot describes the results of the subgroup analysis by ANCA serotype and AAV subtype on the association between RTX use and complete remission. We depict the odds ratio for achieving remission with RTX use. ANCA, antineutrophil cytoplasmic antibody; AAV, ANCA-associated vasculitis; RTX, rituximab
Sensitivity analysis
Using 1:1 propensity score matching, 33 patients from the RTX and non-RTX groups were identified. No significant differences in background characteristics were observed between the two groups (Supplementary Table 2). The odds ratio for achieving remission in the RTX group was 1.89 (95% CI 0.63–5.99) at 24 weeks and 5.2 (95% CI 1.32–26.28) at 48 weeks.
In the complete-case analysis, the odds ratio for achieving remission was 1.54 (95% CI 0.48–5.21) at 24 weeks and 4.10 (95% CI 0.82–25.91) at 48 weeks in multivariate analysis. This result was consistent with the result of the main analysis (Supplementary Table 3).
In the non-RTX group, 16 individuals who received cyclophosphamide, a treatment considered to be as effective as RTX in previous studies, were compared with the RTX group. The baseline characteristics of the patients are described in Supplementary Table 4. Cyclophosphamide was administered intravenously in all but one patient, with a frequency of administration of 1–6. The cumulative dose is detailed in Table 1. A trend toward higher BVAS was observed in cyclophosphamide-treated patients. The proportion of patients achieving CR at week 24 in the RTX- and cyclophosphamide-treated groups was similar (38 [79.2% vs. 12 [75.0%], p = 1.000]) (Supplementary Table 5). However, at week 48, the RTX group had a significantly higher proportion of patients achieving CR.
Discussion
This study explored the effectiveness and safety of RTX in patients with severely relapsed AAV using a multicentre registry database. We found numerically higher CR proportions at weeks 24 and 48 in the RTX group than in the non-RTX group with tolerable safety; however, there was no statistically significant difference in odds ratios at week 24 and the incidence of serious infection for RTX treatment after adjusting for multiple factors.
Previous studies confirmed the effectiveness of RTX in AAV relapse [10, 12]. A recent study including patients from the RITAZERAM trial showed a 90% remission rate after 4 months of RTX treatment in patients who experienced a relapse [10]. In our study, the CR rate in the RTX group was 90.0% at 48 weeks, which is consistent with the results of previous studies. B cell repopulation within 1 year of B cell depletion therapy is known to increase the risk of AAV relapse [17]. Furthermore, relapses after RTX treatment are associated with low plasma RTX concentration [19]. Therefore, our findings are consistent with this pathophysiological understanding.
This study has three strengths compared with previous studies. First, our study analysed outcomes at 24 and 48 weeks, providing a more extended observation period. This longer observation period effectively demonstrated the long-term effectiveness of RTX treatment. Second, we focused on patients experiencing severe relapses with a higher median BVAS than those in previous studies. Because RTX is typically recommended for severe cases, our study provides valuable insights into this specific patient group [15]. Third, we obtained detailed data on disease activity, laboratory data and treatment drugs from our registry compared to a previous study [13]. Our data showed that the incidence of serious infections did not increase in patients receiving RTX, suggesting a potential risk–benefit advantage. Therefore, we believe that our results will provide useful information for physicians to determine an appropriate treatment strategy for relapsed AAV cases.
The present study has several limitations. First, it was a multicentre, retrospective study in which patient treatment was guided by the treating physician’s judgment, which could have led to confounding by indication. The RTX group demonstrated a higher prevalence of PR3-ANCA and GPA, with notable pulmonary and nerve involvements, whereas the non-RTX group exhibited a greater incidence of renal involvement. It is unknown which specific organ involvement responds best to RTX treatment; however, the differences in organ involvement between the groups might have contributed to the observed differences in outcomes. However, we observed improved therapeutic outcomes in the RTX group, even with a higher proportion of patients with PR3-ANCA and GPA. Second, the relatively small sample size may have resulted in the lack of statistically significant differences in certain outcomes. Despite the limited data, this study indicates a positive effect of RTX on relapsed AAV over a prolonged observation period. The larger the sample size, the more likely the detection of statistical differences. Third, although our study only included patients who experienced severe relapse, many of those who did not receive RTX treatment did not receive cyclophosphamide recommended for severe relapse. This may have led to suboptimal treatment in the control group, potentially overestimating the effectiveness of RTX treatment. Consequently, we compared the outcomes between the RTX- and cyclophosphamide-treated groups. We found that the proportion of patients with CR was higher in the RTX group, which is consistent with our main analysis. Finally, because this was a retrospective study, we could not standardize the frequency of RTX administration; five patients received one dose of RTX and six received two doses. The typical dose in Japan is 375 mg/m2 of body surface area. The inclusion of patients who received one or two doses of RTX might have led to an underestimation of the therapeutic effect owing to suboptimal treatment intensity. The effectiveness of RTX even under these conditions validates the findings of this study.
This study revealed the potential effectiveness and safety of RTX compared with those of traditional immunosuppressive therapy in patients with severely relapsed AAV. Although no statistically significant difference was observed at 24 weeks, clinicians may safely induce CR in a larger number of patients using RTX, irrespective of the ANCA serotype or AAV subtype. Thus, further prospective, large-scale studies are warranted.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Kitching AR, Anders HJ, Basu N et al (2020) ANCA-associated vasculitis. Nat Rev Dis Primers 6:71. https://doi.org/10.1038/s41572-020-0204-y
Salama AD (2020) Relapse in anti-neutrophil cytoplasm antibody (ANCA)–associated vasculitis. Kidney Int Rep 5:7–12. https://doi.org/10.1016/j.ekir.2019.10.005
Nakazawa D, Masuda S, Tomaru U, Ishizu A (2019) Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat Rev Rheumatol 15:91–101. https://doi.org/10.1038/s41584-018-0145-y
McClure M, Gopaluni S, Jayne D, Jones R (2018) B cell therapy in ANCA-associated vasculitis: current and emerging treatment options. Nat Rev Rheumatol 14:580–591. https://doi.org/10.1038/s41584-018-0065-x
Nagasaka K, Amano K, Dobashi H et al (2023) Nation-wide cohort study of remission induction therapy using rituximab in Japanese patients with antineutrophil cytoplasmic antibody–associated vasculitis: effectiveness and safety in the first 6 months. Mod Rheumatol 33:1117–1124. https://doi.org/10.1093/mr/roac150
Jones RB, Tervaert JWC, Hauser T et al (2010) Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 363:211–220. https://doi.org/10.1056/NEJMoa0909169
Thietart S, Karras A, Augusto JF et al (2022) Evaluation of rituximab for induction and maintenance therapy in patients 75 years and older with antineutrophil cytoplasmic antibody–associated vasculitis. JAMA Netw Open 5:e2220925. https://doi.org/10.1001/jamanetworkopen.2022.20925
Timlin H, Lee SM, Manno RL, Seo P, Geetha D (2015) Rituximab for remission induction in elderly patients with ANCA-associated vasculitis. Semin Arthritis Rheum 45:67–69. https://doi.org/10.1016/j.semarthrit.2015.02.005
Aqeel F, Xu L, Salas A et al (2023) Outcomes of remission induction therapy for ANCA-associated vasculitis in the elderly. Clin Rheumatol 42:2427–2435. https://doi.org/10.1007/s10067-023-06644-2
Smith RM, Jones RB, Specks U et al (2020) Rituximab as therapy to induce remission after relapse in ANCA-associated vasculitis. Ann Rheum Dis 79:1243–1249. https://doi.org/10.1136/annrheumdis-2019-216863
Miloslavsky EM, Specks U, Merkel PA et al (2014) Rituximab for the treatment of relapses in antineutrophil cytoplasmic antibody–associated vasculitis. Arthritis Rheumatol 66:3151–3159. https://doi.org/10.1002/art.38788
Jones RB, Ferraro AJ, Chaudhry AN et al (2009) A multicenter survey of rituximab therapy for refractory antineutrophil cytoplasmic antibody–associated vasculitis. Arthritis Rheum 60:2156–2168. https://doi.org/10.1002/art.24637
Ishikawa Y, Tokutsu K, Nakayamada S et al (2024) Short-term effectiveness and safety of rituximab versus cyclophosphamide for life-threatening ANCA-associated vasculitis: a propensity score analysis of the real-world nationwide database. Ann Rheum Dis 83:103–111. https://doi.org/10.1136/ard-2023-224472
Jennette JC, Falk RJ, Bacon PA et al (2013) 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum 65:1–11. https://doi.org/10.1002/art.37715
Hellmich B, Sanchez-Alamo B, Schirmer JH et al (2024) EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann Rheum Dis 83:30–47. https://doi.org/10.1136/ard-2022-223764
Mukhtyar C, Lee R, Brown D et al (2009) Modification and validation of the birmingham vasculitis activity score, 3rd version. Ann Rheum Dis 68:1827. https://doi.org/10.1136/ard.2008.101279
Alberici F, Smith RM, Jones RB et al (2015) Long-term follow-up of patients who received repeat-dose rituximab as maintenance therapy for ANCA-associated vasculitis. Rheumatology (Oxford) 54:1153–1160. https://doi.org/10.1093/rheumatology/keu452
Morgan MD, Szeto M, Walsh M et al (2017) Negative anti-neutrophil cytoplasm antibody at switch to maintenance therapy is associated with a reduced risk of relapse. Arthritis Res Ther 19:129. https://doi.org/10.1186/s13075-017-1321-1
Khoudour N, Delestre F, Jabot-Hanin F et al (2023) Association between plasma rituximab concentration and the risk of major relapse in antineutrophil cytoplasmic antibody–associated vasculitides during rituximab maintenance therapy. Arthritis Rheumatol 75:2003–2013. https://doi.org/10.1002/art.42556
Acknowledgements
We thank all staff who treated the enrolled patients at the collaborating institutions. We also thank N. Oka (Hiroshima Red Cross Hospital and Atomic-bomb Survivors Hospital, Hiroshima, Japan) and S. Masuda and A. Yorishima (Hiroshima Prefectural Hospital, Hiroshima, Japan) for helping us collect information on patients with AAV at our institution. We also extend our gratitude to Editage (www.editage.jp) for English language editing.
Funding
Open Access funding provided by Hiroshima University.
Author information
Authors and Affiliations
Consortia
Contributions
All authors have approved the final version of the manuscript and take full responsibility for the integrity of all aspects of the work. Furthermore, all authors have made substantial contributions to this paper, detailed as follows: Genki Kidoguchi: conceptualization, methodology, software, formal analysis, writing — original draft, visualization. Yusuke Yoshida: conceptualization, data curation, methodology, data acquisition, writing — original draft, writing — reviewing and editing. Hirofumi Watanabe: writing — reviewing and editing. Tomohiro Sugimoto: data acquisition, writing — reviewing and editing. Sho Mokuda: writing — reviewing and editing. Takashi Kida: data curation, data acquisition, project administration, writing — reviewing and editing. Nobuyuki Yajima: data curation, writing — reviewing and editing. Satoshi Omura: data acquisition, writing — reviewing and editing. Daiki Nakagomi: data acquisition, writing — reviewing and editing. Yoshiyuki Abe: data acquisition, writing — reviewing and editing. Masatoshi Kadoya: data acquisition, writing — reviewing and editing. Naoho Takizawa: data acquisition, writing — reviewing and editing. Atsushi Nomura: data acquisition, writing — reviewing and editing. Yuji Kukida: data acquisition, writing — reviewing and editing. Naoya Kondo: data acquisition, writing — reviewing and editing. Yasuhiko Yamano: data acquisition, writing — reviewing and editing. Takuya Yanagida: data acquisition, writing — reviewing and editing. Koji Endo: data acquisition, writing — reviewing and editing. Kiyoshi Matsui: data acquisition, writing — reviewing and editing. Tohru Takeuchi: data acquisition, writing — reviewing and editing. Kunihiro Ichinose: data acquisition, writing — reviewing and editing. Masaru Kato: data acquisition, writing — reviewing and editing. Ryo Yanai: data acquisition, writing — reviewing and editing. Yusuke Matsuo: data acquisition, writing — reviewing and editing. Yasuhiro Shimojima: data acquisition, writing — reviewing and editing. Ryo Nishioka: data acquisition, writing — reviewing and editing. Ryota Okazaki: data acquisition, writing — reviewing and editing. Tomoaki Takata: data acquisition, writing — reviewing and editing. Takafumi Ito: data acquisition, writing — reviewing and editing. Mayuko Moriyama: data acquisition, writing — reviewing and editing. Ayuko Takatani: data acquisition, writing — reviewing and editing. Yoshia Miyawaki: data acquisition, writing — reviewing and editing. Toshiko Ito—Ihara: project administration, writing — reviewing and editing. Takashi Kawaguchi: resources, software, writing — reviewing and editing. Yutaka Kawahito: project administration, writing — reviewing and editing. Shintaro Hirata: writing — reviewing and editing, supervision. All authors have given their final approval of the version to be published and agree to be responsible for all aspects of the work.
Corresponding author
Ethics declarations
Ethics statement
Ethics approval was granted by the Ethics Committee for Epidemiological Research of Hiroshima University (approval number: E-2021–2465). This study was conducted in accordance with the ethical standards of the Declaration of Helsinki, and the requirement for written consent was waived because of the retrospective nature of the study. Approval dates and names of the ethics committees at participating centres are provided in Supplemental Table 6.
An abstract of the data from this study, limited to older individuals, has been published in the “2023 EULAR Congress Abstract Book”. Kidoguchi G, Yoshida Y, Watanabe H et al. (2023) AB0786 induction therapy with rituximab enables sustained remission in elderly patients with relapsed ANCA-associated vasculitis: a retrospective analysis from J-CANVAS, a Japanese multicentre-cohort. Ann Rheum Dis 82:1603–1604. https://doi.org/10.1136/annrheumdis-2023-eular.2439.
Conflict of interest
We disclose the following conflicts of interest with pharmaceutical companies promoting RTX or RTX biosimilars as outlined below. Yusuke Yoshida: speaker fees — Chugai. Takashi Kida: speaker fees — Chugai. Daiki Nakagomi: scholarship donations, speaker fees — Chugai. Naoho Takizawa: speaker fees — Chugai, Kyowa Hakko Kirin, Pfizer. Yuji Kukida: speaker fees — Chugai, Pfizer. Kiyoshi Matsui: research grants — Chugai. Masaru Kato: speaker fees — Chugai, Pfizer. Tomoaki Takata: speaker fees — Chugai; scholarship donations, speaker fees — Kyowa Hakko Kirin. Takafumi Ito: speaker fees — Chugai, Kyowa Hakko Kirin. Takashi Kawaguchi: speaker fees, consultancy — Chugai; speaker fees — Kyowa Hakko Kirin. Yutaka Kawahito: speaker fees, research grants — Chugai; speaker fees — Pfizer. Shintaro Hirata: speaker fees, consultancy fees, research grants — Chugai, Pfizer and additional relationships, including speaker fees, consultancy fees, research grants and honoraria with AbbVie; Asahi — Kasei Pharma; Astellas; AstraZeneca; Ayumi; Bristol Myers Squibb; Boehringer Ingelheim; Daiichi — Sankyo; Eisai; Gilead Sciences; GlaxoSmithKline; Eli Lilly; Janssen; Nippon Shinyaku; Novartis; Otsuka; Taisho; Tanabe — Mitsubishi; and UCB.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kidoguchi, G., Yoshida, Y., Watanabe, H. et al. Effectiveness and safety of rituximab in severely relapsed antineutrophil cytoplasmic antibody–associated vasculitis: a retrospective analysis of a Japanese multicentre cohort from the J-CANVAS. Clin Rheumatol (2024). https://doi.org/10.1007/s10067-024-07096-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10067-024-07096-y