Abstract
Background
Relapse of disease is frequent in anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV). It is unclear whether persistent ANCA when starting maintenance therapy increases the risk of relapse. We examined the association between ANCA status and relapse in two randomised controlled trials.
Methods
ANCA-positive patients in two trials, CYCLOPS and IMPROVE, were switched from cyclophosphamide to maintenance therapy after achieving clinical remission. We classified patients as being either ANCA-positive or ANCA-negative at the time they started maintenance therapy. We compared the risk of relapse in ANCA-positive and ANCA-negative patients.
Results
Of 252 patients included, 102 (40%) experienced at least one relapse during the follow-up period. At the time of the switch from induction to maintenance therapy, 111 were ANCA-positive, of whom 55 (50%) relapsed, compared to 141 patients who were ANCA-negative, of whom 47 (33%) relapsed. In multivariable time-to-event analysis, a reduced risk of relapse was associated with having become ANCA-negative at the time of switching to maintenance therapy (hazard ratio 0.63, 95% confidence interval 0.42–0.95; p = 0.026). In addition, initial proteinase 3 (PR3)-ANCA, younger age, lower serum creatinine, pulsed cyclophosphamide for remission induction, and mycophenolate mofetil for remission maintenance were all associated with an increased risk of relapse.
Conclusions
Becoming ANCA-negative before the switch to maintenance is associated with a reduced risk of relapse.
Trial registration
CYCLOPS: ClinicalTrials.gov, NCT00430105. Registered retrospectively on 31 January 2007. IMPROVE: ClinicalTrials.gov, NCT00307645. Registered retrospectively on 27 March 2006.
Similar content being viewed by others
Background
Granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) are potentially life-threatening inflammatory diseases. Whereas historically they were almost uniformly fatal, 5-year survival is now 78% but is often characterised by frequent relapses which are associated with significant morbidity and mortality [1,2,3,4,5].
Previous studies have identified several factors associated with an increased risk of relapse including the presence of circulating proteinase 3 (PR3)-specific, as opposed to myeloperoxidase (MPO)-specific, anti-neutrophil cytoplasm antibodies (ANCA) at the time of disease diagnosis [6, 7]. The role of ANCA testing after diagnosis in predicting relapse has been the subject of many previous studies, although a recent meta-analysis concluded that persistence or a rise in ANCA titre (irrespective of specificity) during remission was only modestly predictive of relapse [8].
Previous studies suggested that the persistence of PR3-ANCA, but not MPO-ANCA, at the time of discontinuing cyclophosphamide and switching to maintenance therapy is associated with an increased risk of relapse [7, 9]. We used long-term follow-up data from two of the previous European Vasculitis Study Group (EUVAS) clinical trials, CYCLOPS and IMPROVE, to identify whether persistent ANCA positivity at the time of discontinuing cyclophosphamide and switching to maintenance therapy is associated with an increased risk of relapse in patients with moderate-to-severe ANCA-associated vasculitis (AAV) treated with cyclophosphamide as initial remission induction therapy [10, 11].
Methods
Patient population and trial protocols
The trial databases of two EUVAS trials (CYCLOPS [10] and IMPROVE [11]) and long-term follow-up data from the CYCLOPS trial cohort were examined [12].
Briefly, CYCLOPS compared pulse cyclophosphamide versus daily oral cyclophosphamide for inducing remission in 149 patients with newly diagnosed generalised AAV from 42 centres in 13 European countries and Mexico between 1998 and 2002. Patients received cyclophosphamide until 3 months after achieving remission (maximum 12 months) and then converted to azathioprine for maintenance therapy. All patients received prednisolone, initially at 1 mg/kg body weight per day tapered to 12.5 mg per day at the end of month 3 and to 5 mg per day by 18 months.
IMPROVE compared mycophenolate mofetil (MMF) to azathioprine for maintenance of remission in AAV following remission induction in 156 patients with newly diagnosed AAV from 42 centres in 11 European countries between 2002 and 2004. Patients received either daily oral or pulsed cyclophosphamide according to investigator preference until remission or a maximum of 6 months following which they were randomised to azathioprine or MMF maintenance therapy. Both azathioprine and MMF were withdrawn at 42 months. Prednisolone was initially given at 1 mg/kg/day tapering to 5 mg/day after 12 months and discontinued at 24 months [11].
For this post-hoc study, patients who were ANCA-positive at trial entry and achieved remission following induction therapy with cyclophosphamide were included. Cases for which the duration of cyclophosphamide therapy and the time of switching to maintenance therapy could not be determined were excluded.
Data collection
Patients in the CYCLOPS trial were initially followed-up for 18 months. Subsequently, long-term follow-up data were collected using physician-completed standardised data abstraction forms [12]. The following data were collected and used in this study: age and sex at diagnosis, ANCA specificity by enzyme linked immunosorbent assay (ELISA) at diagnosis and whether positive or negative by ELISA at the switch to maintenance therapy, time to clinical remission (as defined in the original trial protocols: the complete absence of clinical disease activity using the Birmingham Vasculitis Activity Score (BVAS) item list and supported by a normal serum C-reactive protein (CRP) concentration [13, 14]) and conversion to maintenance therapy, serum creatinine concentration at diagnosis, clinical diagnosis (GPA vs MPA/renal limited vasculitis), induction cyclophosphamide regime (pulse or daily oral cyclophosphamide), and initial maintenance therapy received. All ANCA assays were performed in local centres according to local protocols and reported as present or absent according to local upper limits of normal. Patients who were positive for both PR3- and MPO-ANCA on ELISA were classified as PR3-ANCA as previous studies have suggested that the presence or absence of PR3-ANCA is a significant predictor of relapse [15, 16].
Renal limited vasculitis was included with MPA based on findings of previous studies [8, 17].
The primary outcome for this study was first relapse. Relapses were defined in the trial protocols as a new occurrence or re-occurrence of major or minor organ involvement attributable to active vasculitis supported by exacerbation of at least two constitutional symptoms and a rise in CRP [13, 14].
Statistical analyses
Categorical data are summarised as frequencies (%) and compared using Fisher’s exact test. Continuous measurements are expressed as medians (25th to 75th percentile) and compared using Mann-Whitey U tests. Multivariate binary logistic regression analysis was used to assess the independence of factors associated with remaining ANCA-positive or becoming ANCA-negative and is reported as the hazard ratio (HR) with 95% confidence interval (CI).
Relapse-free survival time was calculated from the point of remission to first relapse and censored for death or end of follow-up. Univariate Cox regression survival analysis was performed and reported as HR with 95% CI. Multivariable survival analysis was performed using Cox regression models containing all exposures listed above except clinical diagnosis. Clinical diagnosis was excluded from the multivariable models since PR3-ANCA specificity has previously been shown to be a better predictor of relapse than disease phenotype [18]. Confirmation that all covariates included in the Cox regression survival analyses met the proportional hazards assumption was assessed graphically using log (–log) survival curves and plotting partial residuals against time. To determine whether the risk of effect of ANCA status at time to switching to maintenance therapy on risk of relapse was similar for both MPO-ANCA and PR3-ANCA, a Cox regression model was constructed including both initial specificity, ANCA status at switch, and an interaction term between the two. The Nagelkerke pseudo R 2 value for the goodness-of-fit was calculated for two multivariable models, one including all covariates and a second with ANCA status at switch to maintenance therapy removed [19]. Statistical significance was defined as a p value less than 0.05. All analyses were carried out using IBM SPSS for Windows version 20.
Results
Patients
Two hundred and fifty-two patients met the entry criteria for this study. The median length of follow-up was 4 (3.5–4.4) years with a total of 956 patient-years of follow-up. One hundred and two patients experienced at least one relapse during follow-up and 11 died without experiencing a relapse. Other demographic and clinical data, including ANCA status, is shown in Table 1.
The associations between patient characteristics and ANCA status at the switch to maintenance therapy are shown in Table 1. In a multivariate binary logistic regression analysis patients who remained ANCA-positive were younger (HR 0.79 (0.65–0.97) per decade; p = 0.02), had lower serum creatinine at entry (HR 0.89 (0.81–0.98) per 50 μmol/L; p = 0.013), and took longer to achieve remission than those who became ANCA-negative (HR 1.29 (1.08–1.54) per month; p = 0.005). There was no difference in initial induction regime or whether patients were more likely to subsequently receive MMF or azathioprine as remission maintenance agents.
Univariate Cox regression survival analysis confirmed that remaining ANCA-positive at the time of switching to maintenance therapy was associated with an increased risk of relapse (Table 2). The sensitivity, specificity, and positive and negative predictive values of positive ANCA for relapse and negative ANCA for no relapse are shown in Table 3.
The median time to relapse was 19 (8–33) months for patients who were ANCA-positive at the switch to maintenance therapy and 23 (14–37; p = 0.2) months for patients who were ANCA-negative at the switch to maintenance therapy. Factors associated with an increased risk of relapse were PR3-ANCA, younger age, lower serum creatinine, GPA as a diagnosis, pulsed intravenous cyclophosphamide, and receiving MMF maintenance therapy.
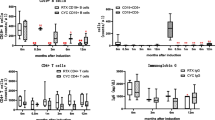
A graphical representation of the effect of ANCA positivity at the time of switching to maintenance therapy for all patients, and for MPO-ANCA and PR3-ANCA patients, is shown in Fig. 1.
Kaplan-Meier survival curves demonstrating the effect of ANCA status at the time of switching to maintenance therapy on all patients (a), and patients who were initially either PR3-ANCA-positive (b) or MPO-ANCA-positive (c). The numbers remaining at risk in the ANCA-positive and ANCA-negative groups at each time point are shown below the appropriate survival curve. ANCA anti-neutrophil cytoplasm antibodies, MPO, myeloperoxidase, PR3 proteinase 3
Prior to constructing the full multivariable Cox regression survival model we tested whether there was an interaction between ANCA specificity at trial entry and ANCA status at switching to maintenance therapy. The hazard ratio for the interaction term was close to 1 and not statistically significant (HR 0.96 (0.40–2.27; p = 0.92)) suggesting that there was no difference in the risk conferred by MPO-ANCA and PR3-ANCA status at switching to maintenance therapy. Two multivariable Cox regression survival models were constructed: one included all covariates except ANCA status at the time of switching to maintenance therapy (not shown); the second additionally included ANCA status at the time of switching to maintenance therapy (Table 4). In both models, PR3-ANCA, pulsed intravenous cyclophosphamide induction therapy, MMF maintenance therapy, and younger age were associated with an increased risk of relapse. There was no increased risk of relapse associated with patient gender or age. In the second model, remaining ANCA-positive at the time of switching to maintenance therapy was associated with an increased risk of relapse and the addition of this variable had little effect on the hazard ratios for relapse associated with the other covariates in the model. The addition of ANCA status at switching to maintenance therapy improved the fit of the model from a pseudo R 2 value of 0.24 to 0.27.
Discussion
Previous studies have consistently demonstrated a higher risk of relapse for patients with PR3-ANCA (or cytoplasmic ANCA (cANCA)) at diagnosis compared to MPO-ANCA (or perinuclear ANCA) [17, 18, 20,21,22]. In this study we addressed the issue of whether persistent ANCA at the time of switching to maintenance therapy was associated with an increased risk of relapse. We demonstrated that persistent ANCA at the switch to maintenance therapy is associated with an increased risk of relapse. In multivariable analysis, this association remained significant after adjusting for initial cyclophosphamide therapy regimen, maintenance therapy, age, and renal function. When we compared two multivariable models of the risk of relapse, the model including ANCA status at the time of switching to maintenance therapy gave a better fit to the observed risk of relapse than the model not including ANCA status at the time of switching to maintenance therapy.
The few studies that have previously examined this issue were limited by smaller sample size and analyses that were not adjusted for other known risk factors for relapse. These studies suggested that persistent cANCA by indirect immunofluorescence (IIF) was associated with an increased risk of relapse compared to patients in whom ANCA re-occurred following the switch to maintenance therapy or who were persistently negative in remission [7, 23]. One recent underpowered retrospective study compared the relapse rate of patients who were cANCA-positive or cANCA-negative at the time of remission and found no significant difference in relapse risk [24]. The significance of persistent MPO-ANCA at the time of switch to maintenance therapy was investigated in 62 Japanese patients with MPA and was not found to be associated with an increased risk of relapse, although several studies have reported an increased risk of relapse associated with an increase in MPO-ANCA titre once in remission [9, 25]. Rituximab is now increasingly being used for both remission induction and maintenance in AAV. Although several trials have reported on re-occurrence of ANCA after initial remission induction as a risk factor for relapse, none have reported on the ANCA status at the time of starting maintenance therapy as a risk factor for relapse [26,27,28]. We tested the overall effect of remaining ANCA-positive at switching to maintenance therapy and also looked for evidence of an interaction between ANCA specificity and ANCA status at switching. There was no evidence of an interaction between these two variables, suggesting that the increased risk of relapse observed in patients remaining ANCA-positive was true for both MPO-ANCA- and PR3-ANCA-positive patients (Fig. 1).
Several factors that are recorded during routine clinical practice have now been identified that associate with an increased risk of relapse in patients with AAV: PR3-ANCA at diagnosis, persistent ANCA at switch to maintenance therapy, better renal function, younger age, pulsed cyclophosphamide for remission induction therapy, MMF for maintenance therapy, corticosteroid withdrawal, and cardiovascular system involvement [11, 12, 17, 20, 29]. Currently there is no clear evidence base to support decision making when considering maintenance or discontinuation of immunosuppression once a patient has been in remission for a period of time, although a recent meta-analysis suggested that patients should receive at least 18 months of corticosteroids to reduce the risk of subsequent relapse [29]. This study provides additional clinical information that can inform the discussion with an individual patient about the risk of relapse when planning maintenance immunosuppression therapy, although it is not possible to conclusively identify those patients who will or will not subsequently relapse—only those with a greater or lesser risk of relapse.
It is interesting to note that the patients who remained ANCA-positive at switching to maintenance therapy took longer to achieve remission than the patients who had become ANCA-negative. In the absence of hard objective measures of disease activity for AAV it is not possible to determine whether this is a real association or whether, because the physicians managing patients in the original trials were not blinded to treatment or ANCA status, knowledge of these factors influenced a physician’s judgement as to when patients entered remission and were switched to maintenance therapy. The same factors could also potentially influence the physician’s judgement as to whether symptoms represented relapse.
Patients in the CYCLOPS trial received cyclophosphamide for a longer period than the patients in the IMPROVE trial, and several CYCLOPS patients who were ANCA-positive at remission became ANCA-negative by the time of switching to maintenance therapy. Due to the small numbers of patients affected it is not possible to determine whether the additional cyclophosphamide received had a significant effect on the risk of relapse.
We are not able to determine from this study the role that ANCA plays in the development of relapse. We do not know how many of the patients who subsequently relapsed remained persistently ANCA-positive and how many of the patients who relapsed were ANCA-positive at the time of relapse. It is interesting to speculate whether ANCA positivity at the time of switching to maintenance therapy indicates a direct pathogenic role for ANCA in the re-occurrence of disease activity or whether it is a biomarker for continued lack of tolerance against the autoantigens and immunological resistance to treatment.
This study is limited in that the data are derived from two clinical trials. These studies were not designed to investigate whether remaining ANCA-positive at time of disease remission increases the risk of disease relapse. We also do not have data on how long the patients in this cohort remained on maintenance immunosuppressive therapy nor can we investigate the effect of cumulative immunosuppression and corticosteroids on relapse risk. However, it provides additional evidence that allows better relapse-risk assessment for the individual patient. It is larger than previous studies which have also suggested that ANCA positivity at the time of remission increases relapse risk and has demonstrated that this is true in multivariable analysis.
It is also important to point out that patients included in this study all received cyclophosphamide induction therapy and all but four received azathioprine or MMF maintenance therapy; thus, these results may not be applicable to patients who have received rituximab or additional plasma exchange as part of their remission induction regime.
Conclusions
The findings of this study provide useful information for the clinician to help stratify patients for their future risk of relapse. Further work is needed in understanding why persistent ANCA at the time of switching is associated with an increased risk of relapse and whether more detailed immunophenotyping of these patients may reveal new therapeutic targets and improved understanding of the mechanisms of AAV disease. In future this may allow us to develop trials specifically targeting new or additional therapy at patients at high risk of relapse to improve outcomes.
Abbreviations
- AAV:
-
Anti-neutrophil cytoplasm antibody-associated vasculitis
- ANCA:
-
Anti-neutrophil cytoplasm antibodies
- BVAS:
-
Birmingham Vasculitis Activity Score
- cANCA:
-
Cytoplasmic anti-neutrophil cytoplasm antibodies
- CI:
-
Confidence interval
- CRP:
-
C-reactive protein
- CYCLOPS:
-
Pulse versus continuous cyclophosphamide for induction of remission in ANCA-associated vasculitides
- ELISA:
-
Enzyme-linked immunosorbent assay
- EUVAS:
-
European Vasculitis Society
- GPA:
-
Granulomatosis with polyangiitis
- HR:
-
Hazard ratio
- IIF:
-
Indirect immunofluorescence
- IMPROVE:
-
Mycophenolate mofetil versus azathioprine for maintenance therapy in ANCA associated systemic vasculitis
- MMF:
-
Mycophenolate mofetil
- MPA:
-
Microscopic polyangiitis
- MPO:
-
Myeloperoxidase
- PR3:
-
Proteinase 3
References
Booth AD, Almond MK, Burns A, Ellis P, Gaskin G, Neild GH, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis. 2003;41:776–84.
Eriksson P, Jacobsson L, Lindell A, Nilsson JA, Skogh T. Improved outcome in Wegener's granulomatosis and microscopic polyangiitis? A retrospective analysis of 95 cases in two cohorts. J Intern Med. 2009;265:496–506.
Corral-Gudino L, Borao-Cengotita-Bengoa M, Del Pino-Montes J, Lerma-Marquez JL. Overall survival, renal survival and relapse in patients with microscopic polyangiitis: a systematic review of current evidence. Rheumatology (Oxford). 2011;50:1414–23.
Flossmann O, Berden A, de Groot K, Hagen C, Harper L, Heijl C, et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis. 2011;70:488–94.
Slot MC, Tervaert JW, Franssen CF, Stegeman CA. Renal survival and prognostic factors in patients with PR3-ANCA associated vasculitis with renal involvement. Kidney Int. 2003;63:670–7.
Sanders JS, Huitma MG, Kallenberg CG, Stegeman CA. Prediction of relapses in PR3-ANCA-associated vasculitis by assessing responses of ANCA titres to treatment. Rheumatology (Oxford). 2006;45:724–9.
Slot MC, Tervaert JW, Boomsma MM, Stegeman CA. Positive classic antineutrophil cytoplasmic antibody (C-ANCA) titer at switch to azathioprine therapy associated with relapse in proteinase 3-related vasculitis. Arthritis Rheum. 2004;51:269–73.
Tomasson G, Grayson PC, Mahr AD, Lavalley M, Merkel PA. Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis—a meta-analysis. Rheumatology (Oxford). 2012;51:100–9.
Wada T, Hara A, Arimura Y, Sada KE, Makino H, Research Group of Intractable Vasculitis, Ministry of Health, Labor, and Welfare of Japan. Risk factors associated with relapse in Japanese patients with microscopic polyangiitis. J Rheumatol. 2012;39:545–51.
de Groot K, Harper L, Jayne DR, Flores Suarez LF, Gregorini G, Gross WL, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. 2009;150:670–80.
Hiemstra TF, Walsh M, Mahr A, Savage CO, de Groot K, Harper L, et al. Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trial. JAMA. 2010;304:2381–8.
Harper L, Morgan MD, Walsh M, Hoglund P, Westman K, Flossmann O, et al. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: long-term follow-up. Ann Rheum Dis. 2012;71:955–60.
Randomised trial of daily oral versus pulse cyclophosphamide as therapy for ANCA-associated systemic vasculitis. http://www.vasculitis.org/images/documents/cyclops.pdf.
International mycophenolate mofetil protocol to reduce outbreaks of vasculitides (IMPROVE). http://www.vasculitis.org/images/documents/improve.pdf.
Mahr A, Katsahian S, Varet H, Guillevin L, Hagen EC, Hoglund P, et al. Revisiting the classification of clinical phenotypes of anti-neutrophil cytoplasmic antibody-associated vasculitis: a cluster analysis. Ann Rheum Dis. 2013;72:1003–10.
Puechal X, Pagnoux C, Perrodeau E, Hamidou M, Boffa JJ, Kyndt X, et al. Long-term outcomes among participants in the WEGENT trial of remission-maintenance therapy for granulomatosis with polyangiitis (Wegener's) or microscopic polyangiitis. Arthritis Rheumatol. 2016;68:690–701.
Walsh M, Flossmann O, Berden A, Westman K, Hoglund P, Stegeman C, et al. Risk factors for relapse of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2012;64:542–8.
Lionaki S, Blyth ER, Hogan SL, Hu Y, Senior BA, Jennette CE, et al. Classification of antineutrophil cytoplasmic autoantibody vasculitides: the role of antineutrophil cytoplasmic autoantibody specificity for myeloperoxidase or proteinase 3 in disease recognition and prognosis. Arthritis Rheum. 2012;64:3452–62.
Nagelkerke N. A note on a general definition of the coefficient of determination. Biometrika. 1991;78:691–2.
Pierrot-Deseilligny Despujol C, Pouchot J, Pagnoux C, Coste J, Guillevin L. Predictors at diagnosis of a first Wegener’s granulomatosis relapse after obtaining complete remission. Rheumatology. 2010;49:2181–90.
Pagnoux C, Hogan SL, Chin H, Jennette JC, Falk RJ, Guillevin L, et al. Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis: comparison of two independent cohorts. Arthritis Rheum. 2008;58:2908–18.
Hogan SL, Falk RJ, Chin H, Cai J, Jennette CE, Jennette JC, et al. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med. 2005;143:621–31.
Thai L, Charles P, Resche-Rigon M, Desseaux K, Guillevin L. Are anti-proteinase-3 ANCA a useful marker of granulomatosis with polyangiitis (Wegener's) relapses? Results of a retrospective study on 126 patients. Autoimmun Rev. 2014;13:313–8.
Sanders JS, de Joode AA, DeSevaux RG, Broekroelofs J, Voskuyl AE, van Paassen P, et al. Extended versus standard azathioprine maintenance therapy in newly diagnosed proteinase-3 anti-neutrophil cytoplasmic antibody-associated vasculitis patients who remain cytoplasmic anti-neutrophil cytoplasmic antibody-positive after induction of remission: a randomized clinical trial. Nephrol Dial Transplant. 2016;31:1453–9.
Terrier B, Saadoun D, Sene D, Ghillani P, Amoura Z, Deray G, et al. Antimyeloperoxidase antibodies are a useful marker of disease activity in antineutrophil cytoplasmic antibody-associated vasculitides. Ann Rheum Dis. 2009;68:1564–71.
Jones RB, Furuta S, Tervaert JW, Hauser T, Luqmani R, Morgan MD, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis: 2-year results of a randomised trial. Ann Rheum Dis. 2015;74:1178–82.
Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaitre O, Cohen P, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014;371:1771–80.
Alberici F, Smith RM, Jones RB, Roberts DM, Willcocks LC, Chaudhry A, et al. Long-term follow-up of patients who received repeat-dose rituximab as maintenance therapy for ANCA-associated vasculitis. Rheumatology (Oxford). 2015;54:1153–60.
Walsh M, Merkel PA, Mahr A, Jayne D. Effects of duration of glucocorticoid therapy on relapse rate in antineutrophil cytoplasmic antibody-associated vasculitis: a meta-analysis. Arthritis Care Res (Hoboken). 2010;62:1166–73.
Acknowledgements
We acknowledge the investigators in the CYCLOPS and IMPROVE clinical trials who generated the data on which this study was based [10, 11].
Funding
The CYCLOPS trial was funded by the European Union (European Community Systemic Vasculitis Trial project, contract BMH1-CT93-1078 and CIPD-CT94-0307, and Associated Vasculitis European Randomised Trial project, contract BMH4-CT97-2328 and IC20-CT97-0019). The funding source had no involvement in the design, conduct, or reporting of the study. The IMPROVE trial received funding from the Cambridge Biomedical Research Centre, the Programme Hospitalier de Recherche Clinique Regionale 2001, and the French Ministry of Health. Hoffman-La Roche Ltd. provided reimbursement for the study drugs to investigators in Germany, France, and Switzerland, for patients recruited in Belgium, France, and Switzerland, and for trial insurance in Germany. MW is supported by the Canadian Institutes of Health Research with a New Investigator Award.
Availability of data and materials
Anonymised data can be made available if requested from the corresponding author.
Authors’ contributions
MDM participated in the current study design, data analysis, and drafting and revision of the manuscript. MS participated in the study design, data analysis, and drafting and revision of the manuscript. MW participated in the design, analysis, and reporting of the IMPROVE trial, design of the current study, data analysis, and revision of the manuscript. DJ participated in the design, conduct, and reporting of the IMPROVE and CYCLOPS trials, design of the current study, and revision of the manuscript. KW participated in the design, analysis, and reporting of the IMPROVE trial, design and conduct of the long-term data collection, and revision of the manuscript. NR participated in the design, conduct, and reporting of the CYCLOPs trial, design of the current study and revision of the manuscript. TFH participated in the design, analysis, and reporting of the IMPROVE trial, design of the current study, and revision of the manuscript. OF participated in the design of the current study, carried out and co-ordinated data-collection, designed the database, and revised the manuscript. AB participated in the current study design, data collection and checking, and revision of the manuscript. PH participated in the design and conduct of the long-term data collection and revision of the manuscript. LH participated in the design, analysis, and reporting of the IMPROVE trial, design of the current study, drafting, and revision of the manuscript. All authors read and approved the final manuscript.
Authors’ information
Not applicable.
Competing interests
DJ has received research grant and consulting fees from Roche/Genentech and consulting fees from GSK and Chemocentryx; LH has received consulting fees from Chemocentryx and lecture fees from Roche; TFH has received consulting fees from Otsuka. The remaining authors declare that they have no competing interests.
Ethics approval and consent to participate
Both trials were conducted according to the 1964 Declaration of Helsinki and subsequent amendments and reported in detail. The full protocols are available at www.vasculitis.org. All participants gave informed consent prior to undergoing any trial procedures. The original trials ran from 1998 to 2004 (CYCLOPS) and 2002 to 2009 (IMPROVE) in 42 centres across 11 countries. At that time there was no requirement to collate a centralised list of all ethical approvals appropriate to each recruiting centre and this was not done. We are not now able to collect this information retrospectively, but all appropriate ethical approvals were in place according to local requirements at the time of the trials and follow-up data collection. Consent for publication is not applicable as only aggregated data are reported.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Morgan, M.D., Szeto, M., Walsh, M. et al. Negative anti-neutrophil cytoplasm antibody at switch to maintenance therapy is associated with a reduced risk of relapse. Arthritis Res Ther 19, 129 (2017). https://doi.org/10.1186/s13075-017-1321-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-017-1321-1