Abstract
Introduction/objectives
Scleroderma is a rare complication in taxanes therapy. Although individual cases of taxanes-induced scleroderma have been reported, the clinical manifestation and treatment outcomes were reviewed and summarized rarely. This study reported a patient who developed diffuse scleroderma and possible scleroderma renal crisis after paclitaxel therapy for ureter cancer.
Method
A PubMed literature review on published cases of taxanes-induced scleroderma up until April 2022 was included for analysis.
Results
The search identified 27 patients with adequate information for analysis. Of the 28 patients, including the one presented here, 22 were female. Peripheral edema was the most common symptom in all but one patient, and often accompanied by erythema in 11. Symptoms usually occurred in half of the patients within the 4th course of treatment. Skin lesions gradually progressed to skin fibrosis, and extended proximally. Internal organ involvements were uncommon. Antinuclear antibody tests were positive occasionally, but anti-Scl70 and anti-centromere usually were negative. Taxanes therapy was discontinued, continued and unavailable in 21, 3, and 4 patients, respectively. Corticosteroids for skin lesions with or without immunosuppressive drugs were given to 15 patients. Of 25 patients with available skin outcomes, 19 improved. There was no significant skin improvement between those who did or did not receive skin treatment (62.5% vs. 75.0%, p = 0.37). Skin usually improved after discontinuing taxanes.
Conclusion
Taxanes-induced scleroderma is different from idiopathic scleroderma. Physicians should be aware of this condition in order to provide early diagnosis and apply appropriate management in order to avoid serious complications from severe skin sclerosis.
Key Points |
• Scleroderma is a rare but unique and serious complication of taxanes therapy • Skin manifestations and distribution are similar to idiopathic scleroderma, but vascular phenomenon, internal organ involvement and scleroderma-associated auto-antibodies are presented rarely. Skin improvement usually occurs shortly after discontinuing taxanes • The role of immunosuppressive therapy in treating taxanes-induced scleroderma is not clear |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paclitaxel and docetaxel, or taxanes compounds, are among the commonly prescribed anti-cancer drugs. Their mechanism enhances polymerization of tubulin to stabilize microtubules, thus preventing microtubules to depolymerize. Microtubules are cytoskeleton structures that are required during cell growth. Taxanes block cells at the G2/M phase of the cell cycle, making them unable to form a normal mitotic apparatus [1]. It has been used in the treatment of various types of malignancies including breast, ovarian, non-small cell lung and gastrointestinal cancer.
The true incidence of cutaneous adverse reaction to taxanes is unknown, but reports have shown between 6 and 81%, according to the literature [2]. A wide range of cutaneous adverse reactions has been reported, including alopecia, onycholysis, mucositis, dysgeusia, peripheral edema, hand-foot syndrome (scaly erythematous lesions of the hands and feet), cutaneous rashes (photosensitive, maculopapular, flexural and intertriginous rashes), subacute cutaneous lupus erythematosus and scleroderma-like skin lesion [2]. Of these, scleroderma-like skin lesion was uncommon. Although cases of taxanes-induced scleroderma have been reported, the clinical manifestation and treatment outcomes were reviewed and summarized rarely [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27].
This study reported a female patient with cancer of the ureter, who developed diffuse scleroderma and possible scleroderma renal crisis (SRC) following paclitaxel treatment. The clinical manifestations and treatment outcomes of taxanes-induced scleroderma also were reviewed.
Materials and methods
Case report
A 68-year-old Thai female patient was consulted at the rheumatology service for progressive skin thickening on the forearms and legs, which had been developing for 3 months. She had presented with suprapubic mass and poorly undifferentiated carcinoma of the distal ureter 2 years previously, and ovarian metastasis was diagnosed. Distal urethrectomy, hysterectomy, and bilateral salpingo-oophorectomy were carried out, but the post-operative computed tomography (CT) scan of the abdomen showed some residual lymphadenopathies, with left common iliac vein invasion. The combination of carboplatin (AUC5) and gemcitabine (1000 mg/m2) was given in 8 cycles. A repeated CT scan of the abdomen, 1 and 6 months after completing a course of chemotherapy, showed progression of the disease with increased size of the lymphadenopathies. Left iliac lymph node irradiation (400 cGray, 5 fractions) was initiated, followed by paclitaxel at 175 mg/m2 every 3 weeks. A repeated CT scan of the abdomen 2 months after three courses of paclitaxel, showed some regression in size of the iliac lymph nodes. During this period, the patient experienced some swelling of both feet, without pain or tenderness. Later, edema at the dorsum of both hands occurred. The edema progressed to induration and thickening of the skin. Rheumatology consultation was performed for possible connective tissue diseases.
Additional history was significant for gastro-esophageal reflux disease (GERD) that occurred during the same period as that for skin thickening. The patient neither drank nor smoked, and denied the use of herbs. There was no family history of malignancies or autoimmune diseases. Her medical history was significant for hypertension and dyslipidemia, and current daily medications were losartan (50 mg) and simvastatin (20 mg).
Physical examination showed an elderly woman, with moderate pallor and fatigued appearance. Thickening of the skin was observed on her face, hands, forearms, left upper arm, both feet, both legs and both thighs, with a modified Rodnan’s skin score of 27 (Fig. 1). Some areas of hyperpigmentation on the thickened skin also were observed. Raynaud’s phenomenon was noted. There were no digital pitting scars, tendon friction rubs, subcutaneous calcifications, muscle weaknesses or arthritis. Bilateral basal crepitation was audible at both basal lungs. Other physical examinations were unremarkable.
Laboratory investigation showed hemoglobin of 7.3 gm/dL (presumably due to underproduction or anemia from chronic disease), white blood counts of 6.1 × 103/mm3, and platelet counts of 346 × 103/mm3. Urine analysis, renal and liver functions, electrolytes, and thyroid functions were normal. Hepatitis B and C profiles were all negative. Anti-nuclear antibody (ANA) was positive at 1:320, speckled pattern. Anti-dsDNA, anti-Scl70 (anti-topoisomerase-I), anti-centromere, anti-SSA, anti-SSB, anti-Sm and anti-RNP antibodies and complements were all normal or negative. Chest X-ray showed minimal interstitial infiltration at both basal lungs. High-resolution CT of the chest showed minimal non-specific interstitial pneumonitis at the base of both lungs. Echocardiography was normal. A skin biopsy was scheduled, but not performed due to the COVID-19 pandemic situation. The diagnosis of diffuse scleroderma induced by paclitaxel was made. Treatment was initiated with daily prednisolone (5 mg), mycophenolate mofetil (2000 mg), hydroxychloroquine (200 mg), amlodipine (10 mg), omeprazole (20 mg) and domperidone (30 mg). Due to the COVID-19 situation, blood transfusion at a local hospital near to home was advised.
Two weeks after receiving 2 units of blood, the patient was admitted without fever to a local hospital because of progressive dyspnea that required intubation. A chest radiograph showed bilateral interstitial infiltrations. She was found to have high blood pressure (158/81 mmHg), progressive anemia (hemoglobin 5.0 gm/dL) and thrombocytopenia (platelet 11.0 × 103 cells/mm3). There was no history of blood losses. Peripheral blood smear showed many schistocytes, some nucleated red blood cells and very few platelets, which was consistent with thrombotic microangiopathy (TMA). Urine analysis showed proteinuria with red blood cells. She also had acute renal insufficiency (creatinine 1.4 mg/dL that progressed to 4.7 mg/dL within 1 week [baseline creatinine before blood transfusion was 0.8–1.0 mg/dL]). As all the clinical features were consistent with SRC, enalapril was given and mycophenolate mofetil discontinued. Unfortunately, the condition of the patient became progressively worse, with multiple site bleeding due to consumptive coagulopathy. The patient and her family denied further investigation and management, and she was discharged in terminal condition and later expired at home.
Review of the literature
A review of the English language literature, using the PubMed database up until April 2022, identified 25 publications with 33 cases of taxanes-induced scleroderma [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. Six cases were excluded from the analysis. The first one was a patient who had history of calcinosis, Raynaud’s Phenomenon, esophageal dysfunction, sclerodactyly and telangiectasia (CREST) syndrome prior to the onset of morphea after docetaxel therapy [4]. The second one received multiple immunosuppressive drugs together with paclitaxel, which made it difficult to conclude that paclitaxel was causal [10]. The third one developed scleroderma after 2 courses of docetaxel and cyclophosphamide therapy, but several clinical data suggested that she might have had systemic sclerosis before developing scleroderma and prior to docetaxel treatment [27]. The other 3 cases were excluded from a series of 5 that received docetaxel or paclitaxel, but with insufficient information for analysis [11]. Therefore, only 28 cases were analyzed, including the one from this report.
Results
Characteristics of the patients studied are shown in Table 1 and Supplementary Table 1. Of the 28 cases, 12 (42.7%) were reported from Japan. Twenty-two female patients (78.6%) had a mean age of 57.1 ± 9.0 years, with breast, ovary and skin malignancies being the 3 most common conditions reported in 13 (46.4%), 4 (14.3%) and 3 (10.7%) of them, respectively. Twelve patients (42.9%) received taxanes (docetaxel, paclitaxel or Nab-paclitaxel) monotherapy, and 16 (57.2%) taxanes in combination with other immunosuppressive or immunomodulator drugs. Of these, 2 patients received Nab-paclitaxel and gemcitabine. The onset of symptoms varied greatly from 10 days to 20 months after taxanes therapy, but 14 cases (50.0%) had onset within the 4th course or 4 months of treatment.
Clinical manifestations and laboratory findings of scleroderma following taxanes therapy are shown in Table 2 and Supplementary Table 2. Swelling or edema of the extremities was the symptom mostly seen in all but one patient (96.4%). Eleven patients (39.3%) were seen to have erythema of the edematous areas, which occasionally mimicked skin infections. Skin lesions involved the lower and upper extremities in 85.7% and 82.1% of the patients, respectively, where the face/neck and truck were involved in 28.6% and 28.6%, respectively. Raynaud’s Phenomenon and telangiectasia were observed in 21.4% and 10.7%, respectively. Tendon friction rubs, cutaneous calcinosis, and digital pitting scars or ulcers were observed occasionally. One patient also had digital gangrene. Nailfold capillaroscopy was abnormal in 3 of the 13 patients (23.1%) determined. Four patients (14.3%) had musculoskeletal involvement (one each in arthralgia, arthritis, myalgia and myositis). Three patients (10.7%) had pulmonary involvement (dyspnea with abnormal carbon monoxide diffusing capacity in 1 and interstitial lung disease (ILD) documented by high resolution computed scan of the chest in 2). Four patients (14.3%) had gastrointestinal involvement (dyspepsia in 2, and GERD in 2). Dysesthesia was noted in 4 patients (14.3%). None had cardiac involvement. Twenty-three patients (82.1%) had skin biopsy confirmation for scleroderma, and one also had concomitant acanthosis nigricans.
Anti-nuclear antibodies (ANA) were the most common autoantibodies identified, and observed in 25.9% of the cases (Table 2). Among the scleroderma autoantibodies, anti-centromere antibodies were observed in 6.3% of those patients tested. Anti-Sjogren’s syndrome A antibodies (anti-SSA) and rheumatoid factor (RF) were positive in 14.3% and 7.7% of cases, respectively, among those tested. Anti-Scl70 or anti-RNA polymerase III (anti-RNAP-III) antibodies, and other autoimmune antibodies were negative generally among those tested. It was interesting that anti-mitochondria and anti-smooth muscle antibodies were positive in 1 of 3 patients tested (33.3%). None of the 6 patients, who had serum complement level determined, showed low complement levels. Nine patients who had thyroid function determined were normal.
Treatment of taxanes-induced scleroderma, and skin and cancer outcomes are shown in Table 3 and Supplementary Table 3. Taxanes therapy was discontinued, continued, and not available information in 21 (75.0%), 3 (10.7%) and 4 (14.3%) patients, respectively. Treatment of skin lesion was available for 15 patients (corticosteroids alone in 3 [20.0%], corticosteroids in combination with other immunosuppressive therapy in 8 [53.3%], immunosuppressive therapy alone in 1 [6.7%], and topical corticosteroids in 3 [20.0%]). D-penicillamine, methotrexate, mycophenolate mofetil, hydroxychloroquine, cyclophosphamide, and psoralen and ultraviolet A [PUVA] were among the immunosuppressive therapies given. The dosage of corticosteroids ranged from 5 to 60 mg/day (prednisolone equivalent). Six patients (21.4%) received vasodilator therapy.
Among the 3 patients who continued taxanes therapy when scleroderma developed, one and one who received topical corticosteroids, and corticosteroids and immunosuppressive drugs, respectively, showed skin improvement. Of the 21 patients who discontinued taxanes therapy, 9 (42.9%) received corticosteroids and/or immunosuppressive drugs, 1 (4.8%) received topical corticosteroids, 1 (4.8%) received vasodilator therapy, and 10 (47.6%) received no medication. Of the 9 patients, who received corticosteroids and/or immunosuppressive drugs, skin edema and thickening were improved, not available, and not mentioned in 5 (55.6%), 3 (33.3%), and 1 (11.1%), respectively. One patient who received topical corticosteroids showed no response, but one who received vasodilator therapy showed improvement in vascular gangrene. Nine of 10 (90.0%) patients who did not receive any medication for systemic sclerosis showed skin improvement. Overall, outcomes of the skin were improved, not improved and unavailable in 19 (67.9%), 6 (21.4%), and 3 (10.7%) cases, respectively, which did not differ between those who did and did not receive treatment for skin sclerosis regardless of their taxanes therapy status (p = 0.37). The skin improvement usually occurred within 4–6 months after taxanes was discontinued.
The outcomes of underlying cancer were available in only 11 patients, whose status of taxanes therapy was discontinued, continued and unavailable in 8 (72.7%), 1 (9.1%), and 2 (18.2%) patients, respectively. Among the 8 patients whose taxanes therapy was discontinued, 3, 1, and 4 had received corticosteroids and/or immunosuppressive drugs, vasodilator therapy and no medication, respectively, and 3 (37.5%) of them died (2 from cancer and 1 from renal failure). Of the remaining 5 patients, 2 had tumor progression, and one each had tumor improvement prior to discontinuation of taxanes, unchanged tumor status and no tumor recurrence. One who continued taxanes therapy (by receiving topical corticosteroid therapy), showed progressive response of the tumor to Nab-paclitaxel and gemcitabine. Of the two patients who had no taxanes therapy status available, one died because of cancer progression and the other from chronic kidney disease. Of the 12 patients who received systemic corticosteroids and/or immunosuppressive therapy, regardless of their taxanes therapy status, only 2 had available cancer outcomes, of which one had no cancer recurrence, and the other was unable to control cancer with non-taxanes anti-cancer drugs.
Discussion
The patient in this study experienced developing edema and thickening of the skin approximately 2 months after paclitaxel therapy for her ureter cancer. The characteristic and distribution of skin lesions were compatible with scleroderma. Although the presence of GERD, interstitial pneumonitis at the base of the lungs, and positive ANA antibodies might support idiopathic scleroderma (or systemic sclerosis) in this patient; the close temporal relationship between the initiation of paclitaxel and onset of skin manifestations, rapid progression of skin lesions, absence of digital pitting scars, digital ulcers, anti-Scl70 and anti-centromere antibodies, as well as other autoantibodies made diagnosis of taxanes-induced scleroderma more likely than idiopathic scleroderma. Unfortunately, the clinical course of this patient progressed to possible SRC and she ended up in terminal condition.
SRC is a serious complication in patients with idiopathic scleroderma. SRC is diagnosed clinically in general by new onset of hypertension accompanied by the presence of proteinuria, hematuria, acute renal failure, and presence of TMA blood picture in the absence of other explainable causes [28, 29]. Early diffuse cutaneous involvement with rapid progression of skin thickening, and the presence of tendon friction rubs, large joint contracture, arthralgias or synovitis, as well as anti-RNAP-III together with corticosteroids therapy (> 15 mg/day of prednisolone) are among the risk factors [28, 29]. Although a case of scleroderma with SRC and TMA following docetaxel therapy was reported recently [27]; the patient developed severe ILD and mega-esophagus after 2 courses of docetaxel treatment for carcinoma of the breast. She later developed SRC and TMA together with positive anti-RNAP-III antibody after high-dose corticosteroids (1 mg/kg/day) for the treatment of ILD, which was clearly a risk factor for SRC. The presence of mega-esophagus suggested that she might have had systemic sclerosis before the onset of taxanes-induced scleroderma, as the development of mega-esophagus needs time.
The presence of acute hypertensive episodes, together with that of acute renal failure, proteinuria, and TMA blood pictures gave a diagnostic challenge for SRC in the presented case. Infection and malignancies are among the common causes of TMA; however, infection was not identified in this case. While the possibility that TMA could be from underlying malignancy in this case, the development of TMA at the time of hypertension and acute renal failure favored TMA as a picture of SRC rather than TMA being secondary to underlying malignancies. Unfortunately, renal biopsy was not performed to confirm renal pathological diagnosis, and anti-RNAP-III antibody was not determined due to unavailability at this institution. Although anti-RNAP-III antibody is a strong risk factor for SRC, its prevalence of 15–52% in patients with SRC had been reported [30, 31]. This wide variation in prevalence in SRC patients might have been related to a wide variation (0–41%) of the prevalence of anti-RNAP-III among various ethnics [32]. It has been noted that among the 28 patients reviewed, anti-RNAP-III antibody was tested or mentioned in only 3, and none were positive. A patient in this review (case No. 19) also had renal failure, and anemia. However, as the cause of anemia and blood smear pictures were not provided, the renal failure was suspected to be from pre-renal causes, and the anti-RNAP-III antibody was negative [19]. Therefore, the authors believed that the presented case had SRC, although it was not confirmed by renal pathology or anti-RNAP-III antibody.
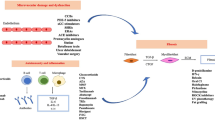
The pathogenesis of taxanes-induced scleroderma is not clearly understood, which might be due to the small number of reported cases. Tsavaris et al. found that an increase in interleukin (IL) -2, IL-6, granulocyte–macrophage colony-stimulating factor (GM-CSF), and interferon-γ (IFNγ) activity led to enhanced peripheral blood mononuclear cells (PBMC), natural killer (NK) cells, and lymphokine activated killer (LAK) cells in breast cancer patients treated with taxanes [33]. Yang et al. also found docetaxel induced skin fibrosis through regulation of type I collagen, fibrinonectin and connective tissue growth factor (CTGF) [26]. In the severe combined immunodeficient (SCID) mouse model, Liu et al. found that high-dose paclitaxel was associated with pro-fibrotic and anti-angiogenetic changes, where low-dose paclitaxel prevented maintenance of the systemic sclerosis phenotype [34]. This effect was mediated by paclitaxel suppressed TGFb/Smad activity and led to lessen fibrosis. Furthermore, Okada et al. found intense versican deposits in the skin of patients with scleroderma following docetaxel therapy [17]. Versican is a large chondroitin sulfate proteoglycan that can hold a large amount of water, which could explain peripheral edema at the early stage of or prior to skin thickening in scleroderma following taxanes therapy. Versican increases inflammation by forming a microcellular environment and functions as a reservoir of cytokines [17]. Takahashi et al. found that Friend leukemia integration-1 (FLI-1) proteins were abundant in dermal microvascular endothelial cells, but decreased markedly in dermal fibroblasts in patients with taxanes-induced scleroderma [20]. This was in contrast to the decrease in FLI-1 in both dermal microvascular endothelial and fibroblast cells observed in patients with systemic sclerosis. These findings indicated that although skin manifestations and cytokine profiles in taxanes-induced scleroderma were somewhat similar to those of idiopathic scleroderma [35, 36], the underlying pathogenesis of these two conditions clearly might be different. Lastly, it is interesting that 12 of the 28 taxanes-induced scleroderma cases were reported from Japan; therefore, the ethnic or genetic background might influence the pathogenesis of the disease. Searching for genetic risk factors for this condition would be of interest.
In general, the clinical syndrome of taxanes-induced scleroderma, particularly the skin thickening and distribution of skin involvement, resembled that seen in idiopathic scleroderma. However, when observed carefully, it clearly had some differences (Table 4). The absence or low prevalence of vascular phenomena including Raynaud’s symptoms, abnormal nailfold capillaroscopy at disease onset, absence or low prevalence of digital pitting scars or digital ulcers, predominant involvement at the lower extremities in the early stage of disease, low prevalence of facial involvement, low prevalence of skin telangiectasia, rapid progression of skin lesions from edematous to sclerotic phase, and absence or low prevalence of musculoskeletal and internal organ involvements, as well as the absence of scleroderma-associated autoantibodies including anti-Scl70, anti-centromere and anti-RNAP (except ANA occasionally observed), clearly made taxanes-induced scleroderma different from idiopathic scleroderma [36]. In terms of response to treatment, the edematous and sclerotic skin that usually resolved shortly after taxanes was discontinued, whether or not the patients received immunosuppressive drugs, clearly differed from that observed in idiopathic scleroderma, which usually took a long period of time to respond. That taxanes-induced scleroderma might be a paraneoplastic syndrome that occurred while being treated with taxanes also could be considered. However, as cancer treatment progressed with tumor response, improvement in clinical scleroderma symptoms would be expected after taxanes therapy. These findings made paraneoplastic syndrome less likely.
Whether a patient with taxanes-induced scleroderma should be treated with corticosteroids or immunosuppressive drugs in a similar way to that for idiopathy scleroderma was an interesting issue. Not only the small number of patients in this review, but also the wide range of dosages and regimens used (either corticosteroids alone or in combination with immunosuppressive drugs), made it difficult to ascertain the treatment results. It was interesting that skin improvement was observed in almost all of the patients who had taxanes therapy discontinued without receiving any treatment for skin lesion. Furthermore, there was no significant difference in improvement of skin lesions between those who did or did not receive treatment for this condition. Only 2 cancer outcomes were available among 12 patients who received systemic corticosteroids or immunosuppressive drug therapy for skin lesions. Therefore, the role of corticosteroids and immunosuppressive therapy for skin lesions in taxanes-induced scleroderma and their effect on cancer was not clear. In addition, certain immunosuppressive drugs, e.g. methotrexate and cyclophosphamide, also have anti-cancer activity. Thus, prescribing them in combination with taxanes or anti-cancer agents should be cautionary as they can potentiate side effects, particularly bone marrow suppression.
Should taxanes therapy be reintroduced and when also are issues for discussion. Although skin improvement usually occurs within 4–6 months after stopping taxanes, one patient had recurrence of scleroderma after taxanes was reintroduced, and skin regression occurred after the second course was discontinued (case no. 12) [12]. As taxanes-induced scleroderma is a serious complication, reintroduction of taxanes-based therapy should be weighted between benefit (e.g., cancer type and staging and their prior response to taxanes compounds) and risk (e.g., severity and rapidity of skin and internal organ involvement in scleroderma). Non-taxanes-based anti-cancer therapy might be another option. Such treatment should be discussed thoroughly between physicians and patients.
Conclusions
Taxanes-induced scleroderma is rare, but a unique and serious complication of taxanes therapy. Skin lesions usually start shortly after taxanes treatment, beginning in the lower extremities with edema evolving into skin thickening and sclerosis, and progressing proximally. Taxanes-induced scleroderma differs from idiopathic scleroderma in that it usually has no vascular phenomenon, absence of internal organ involvement, and lack of specific scleroderma autoantibodies. The majority of patients showed skin improvement after discontinuing taxanes. Physicians should be aware of this condition in order to provide early diagnosis and apply appropriate management to avoid serious complication from severe skin sclerosis.
References
Pazdur R, Kudelka AP, Kavanagh JJ, Cohen PR, Raber MN (1993) The taxoids: paclitaxel (Taxol) and docetaxel (Taxotere). Cancer Treat Rev 19(4):351–386. https://doi.org/10.1016/0305-7372(93)90010-o
Sibaud V, Leboeuf NR, Roche H, Belum VR, Gladieff L, Deslandres M, Montastruc M, Eche A, Vigarios E, Dalenc F, Lacouture ME (2016) Dermatological adverse events with taxane chemotherapy. Eur J Dermatol 26(5):427–443. https://doi.org/10.1684/ejd.2016.2833
Battafarano DF, Zimmerman GC, Older SA, Keeling JH, Burris HA (1995) Docetaxel (Taxotere) associated scleroderma-like changes of the lower extremities. A report of three cases Cancer 76(1):110–115. https://doi.org/10.1002/1097-0142(19950701)76:1%3c110::aid-cncr2820760117%3e3.0.co;2-9
Bouchard SM, Mohr MR, Pariser RJ (2010) Taxane-induced morphea in a patient with CREST syndrome. Dermatol Reports 2(1):e9. https://doi.org/10.4081/dr.2010.e9
Cleveland MG, Ajaikumar BS, Reganti R (2000) Cutaneous fibrosis induced by docetaxel: a case report. Cancer 88(5):1078–1081
De Angelis R, Bugatti L, Cerioni A, Del Medico P, Filosa G (2003) Diffuse scleroderma occurring after the use of paclitaxel for ovarian cancer. Clin Rheumatol 22(1):49–52. https://doi.org/10.1007/s10067-002-0635-8
Kawakami T, Tsutsumi Y, Soma Y (2009) Limited cutaneous systemic sclerosis induced by paclitaxel in a patient with breast cancer. Arch Dermatol 145(1):97–98. https://doi.org/10.1001/archdermatol.2008.532
Kilic MO, Yalaza M, Bilgic CI, Dener C (2015) Docetaxel-induced scleroderma in a breast cancer patient: a case report. J Breast Health 11(2):95–97. https://doi.org/10.5152/tjbh.2015.1879
Hassett G, Harnett P, Manolios N (2001) Scleroderma in association with the use of docetaxel (taxotere) for breast cancer. Clin Exp Rheumatol 19(2):197–200
Farrant PB, Mortimer PS, Gore M (2004) Scleroderma and the taxanes. Is there really a link? Clin Exp Dermatol 29(4):360–362. https://doi.org/10.1111/j.1365-2230.2004.01519.x
Itoh M, Yanaba K, Kobayashi T, Nakagawa H (2007) Taxane-induced scleroderma. Br J Dermatol 156(2):363–367. https://doi.org/10.1111/j.1365-2133.2006.07597.x
Kupfer I, Balguerie X, Courville P, Chinet P, Joly P (2003) Scleroderma-like cutaneous lesions induced by paclitaxel: a case study. J Am Acad Dermatol 48(2):279–281. https://doi.org/10.1067/mjd.2003.30
Lauchli S, Trueb RM, Fehr M, Hafner J (2002) Scleroderma-like drug reaction to paclitaxel (Taxol). Br J Dermatol 147(3):619–621. https://doi.org/10.1046/j.1365-2133.2002.488210.x
Motegi SI, Ishikawa M, Sekiguchi A, Ishikawa O (2019) Nanoparticle albumin-bound paclitaxel- and/or gemcitabine-induced scleroderma accompanied by acanthosis nigricans-like skin changes. Case Rep Dermatol 11(3):273–277. https://doi.org/10.1159/000503271
Ogawa T, Okiyama N, Koguchi-Yoshioka H, Fujimoto M (2017) Taxane-induced scleroderma-like skin changes resulting in gangrene: a case report. J Dermatol 44(4):e54–e55. https://doi.org/10.1111/1346-8138.13569
Ohashi A, Minagawa A, Ashida A, Koga H, Uhara H, Okuyama R (2015) Histopathological improvement of scleroderma induced by paclitaxel in a patient with breast cancer. J Dermatol 42(12):1198–1199. https://doi.org/10.1111/1346-8138.13085
Okada K, Endo Y, Miyachi Y, Koike Y, Kuwatsuka Y, Utani A (2015) Glycosaminoglycan and versican deposits in taxane-induced sclerosis. Br J Dermatol 173(4):1054–1058. https://doi.org/10.1111/bjd.13899
Pedersen JV, Jensen S, Krarup-Hansen A, Riis L (2010) Scleroderma induced by paclitaxel. Acta Oncol 49(6):866–868. https://doi.org/10.3109/02841861003702510
Shibao K, Okiyama N, Maruyama H, Jun-Ichi F, Fujimoto M (2016) Scleroderma-like skin changes occurring after the use of paclitaxel without any chemical solvents: a first case report. Eur J Dermatol 26(3):317–318. https://doi.org/10.1684/ejd.2016.2763
Takahashi T, Asano Y, Ichimura Y, Taniguchi T, Kogure A, Tamaki Z, Takekoshi T, Sugaya M, Sato S (2011) A case of taxane-induced scleroderma: a different expression profile of Fli1 proteins in dermal fibroblasts and microvascular endothelial cells compared with systemic sclerosis. Br J Dermatol 164(6):1393–1395. https://doi.org/10.1111/j.1365-2133.2011.10243.x
Tani N, Sugita K, Yamamoto O (2018) Paclitaxel-related scleredema-like skin changes in a patient with breast cancer. Australas J Dermatol 59(3):e215–e217. https://doi.org/10.1111/ajd.12719
Winkelmann RR, Yiannias JA, DiCaudo DJ, Trotter SC, Farhey Y, Griffing WL, Martorano LM, Winkelmann JC (2016) Paclitaxel-induced diffuse cutaneous sclerosis: a case with associated esophageal dysmotility, Raynaud’s phenomenon, and myositis. Int J Dermatol 55(1):97–100. https://doi.org/10.1111/ijd.12437
Konishi Y, Sato H, Sato N, Fujimoto T, Fukuda J, Tanaka T (2010) Scleroderma-like cutaneous lesions induced by paclitaxel and carboplatin for ovarian carcinoma, not a single course of carboplatin, but re-induced and worsened by previously administrated paclitaxel. J Obstet Gynaecol Res 36(3):693–696. https://doi.org/10.1111/j.1447-0756.2010.01171.x
Verhulst L, Noe E, Morren MA, Verslype C, Van Cutsem E, Van den Oord JJ, De Haes P (2018) Scleroderma-like cutaneous lesions during treatment with paclitaxel and gemcitabine in a patient with pancreatic adenocarcinoma. Review of literature Int J Dermatol 57(9):1075–1079. https://doi.org/10.1111/ijd.14067
Cury-Martins J, Giesen L, Gonzalez S, Molgo M, Sanches JA (2021) Taxane-induced scleroderma. Report of two cases. Rev Med Chil 149(5):807–809. https://doi.org/10.4067/s0034-98872021000500807
Yang JQ, Dou TT, Chen XB, Min M, Cai SQ, Zheng M, Man XY (2017) Docetaxel-induced scleroderma: a case report and its role in the production of extracellular matrix. Int J Rheum Dis 20(11):1835–1837. https://doi.org/10.1111/1756-185X.12697
Debien V, Petitdemange A, Bazin D, Ederle C, Nespola B, Merdji H, Olagne J, Martin T, Guffroy A, Pflumio C (2021) New-onset systemic sclerosis and scleroderma renal crisis under docetaxel. J Scleroderma Relat Disord 6(3):306–310. https://doi.org/10.1177/23971983211007669
Steen VD, Medsger TA Jr (1998) Case-control study of corticosteroids and other drugs that either precipitate or protect from the development of scleroderma renal crisis. Arthritis Rheum 41(9):1613–1619. https://doi.org/10.1002/1529-0131(199809)41:9%3c1613::AID-ART11%3e3.0.CO;2-O
Woodworth TG, Suliman YA, Li W, Furst DE, Clements P (2018) Scleroderma renal crisis and renal involvement in systemic sclerosis. Nat Rev Nephrol 14(2):137. https://doi.org/10.1038/nrneph.2017.183
Codullo V, Cavazzana I, Bonino C, Alpini C, Cavagna L, Cozzi F, Del Papa N, Franceschini F, Guiducci S, Morozzi G, Ruffatti A, Ferri C, Giacomelli R, Matucci-Cerinic M, Valentini G, Montecucco C (2009) Serologic profile and mortality rates of scleroderma renal crisis in Italy. J Rheumatol 36(7):1464–1469. https://doi.org/10.3899/jrheum.080806
Nguyen B, Assassi S, Arnett FC, Mayes MD (2010) Association of RNA polymerase antibodies with scleroderma renal crisis. J Rheumatol 37(5):1068–1069. https://doi.org/10.3899/jrheum.091048 (author reply)
Sobanski V, Dauchet L, Lefevre G, Lambert M, Morell-Dubois S, Sy T, Hachulla E, Hatron PY, Launay D, Dubucquoi S (2014) Prevalence of anti-RNA polymerase III antibodies in systemic sclerosis: new data from a French cohort and a systematic review and meta-analysis. Arthritis Rheumatol 66(2):407–417. https://doi.org/10.1002/art.38219
Tsavaris N, Kosmas C, Vadiaka M, Kanelopoulos P, Boulamatsis D (2002) Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer 87(1):21–27. https://doi.org/10.1038/sj.bjc.6600347
Liu X, Zhu S, Wang T, Hummers L, Wigley FM, Goldschmidt-Clermont PJ, Dong C (2005) Paclitaxel modulates TGFbeta signaling in scleroderma skin grafts in immunodeficient mice. PLoS Med 2(12):e354. https://doi.org/10.1371/journal.pmed.0020354
Raja J, Denton CP (2015) Cytokines in the immunopathology of systemic sclerosis. Semin Immunopathol 37(5):543–557. https://doi.org/10.1007/s00281-015-0511-7
Denton CP, Khanna D (2017) Systemic sclerosis. Lancet 390(10103):1685–1699. https://doi.org/10.1016/S0140-6736(17)30933-9
Acknowledgements
The authors thank Mrs. Waraporn Sukitawut for her secretarial assistance.
Author information
Authors and Affiliations
Contributions
Conceptualization: T. Ketpueak, W. Louthrenoo.
Data collection: W. Chanloung, C. Pongsananurak, K. Na Nan, W. Louthrenoo.
Formal analysis: W. Chanloung, W. Louthrenoo.
Writing — original draft preparation: T. Ketpueak, W. Louthrenoo.
Writing — review and editing: W. Chanloung, K. Na Nan, C. Pongsananurak, N. Kasitanon.
Writing — final manuscript: T. Ketpueak, W. Louthrenoo.
All of the authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical standards
This study was approved by the Ethics Committee of the Faculty of Medicine, Chiang Mai University (no. 8839/2022). It was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate and publish
Written informed consent for data collection and publication was obtained from the patient’s daughter.
Conflict of interest
The authors of this work have nothing to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ketpueak, T., Chanloung, W., Nan, K.N. et al. Paclitaxel-induced diffuse scleroderma with possible scleroderma-renal crisis: a case report and literature review of taxanes-induced scleroderma. Clin Rheumatol 41, 3887–3896 (2022). https://doi.org/10.1007/s10067-022-06364-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-022-06364-z