Abstract
Introduction/Objectives
Ankylosing spondylitis (AS) is a chronic inflammatory immune-mediated condition. We compared AS diagnosis, treatment, and burden in Central Eastern European countries (CEE), where this has been less researched, and the United States (US) from a real-world perspective.
Methods
Point-in-time survey of rheumatologists and their AS patients was conducted in the US (Apr–Oct 2018) and CEE (Aug–Nov 2019) via physician- and patient-completed record forms, including clinical and patient-reported outcomes. Statistical analysis included descriptive statistics, t-tests, Fisher’s exact tests, and generalized linear models.
Results
In total, 487 patients were recruited from 88 rheumatologists in the US and 922 patients from 126 rheumatologists in CEE. Time from onset of symptoms to final AS diagnosis was longer in CEE than the US (4.2 vs 2.7 years, p < 0.05). At diagnosis, a greater use of conventional synthetic disease-modifying antirheumatic drugs (DMARDs) and injected steroids was reported in CEE vs the US (43.7% vs 27.6%, p < 0.05; 19.3% vs 8.7%, p < 0.05). 22.9% of US patients received a biologic DMARD at diagnosis vs 10% of CEE patients (p < 0.05). At current consultation, biologic DMARD use in CEE was lower vs the US (27.9% vs 71.0%, p < 0.05). CEE vs US patients had greater disease activity (mean Bath Ankylosing Spondylitis Disease Activity Index 4.2 vs 3.1, p < 0.05) and worse quality of life (QoL; mean Ankylosing Spondylitis Quality of Life Questionnaire score 6.2 vs 8.4, p < 0.05).
Conclusions
AS patients in CEE vs the US faced slower diagnosis and worse access to biologics, disease activity, and QoL. Whether early access to biologics can improve symptoms, QoL, and daily activities in AS patients in CEE remains to be seen.
Key Points • The study provided evidence on the real-world approach to the diagnosis, treatment, and burden of axSpA (axial spondyloarthritis) in CEE compared with the US. • The study reported patients in CEE experienced longer delays in diagnosis and poorer access to biologics than in the US. • This may have resulted in higher disease activity, greater levels of pain, and poorer outcomes, as reported by patients with axSpA in CEE. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Axial spondyloarthritis (axSpA) is a chronic inflammatory immune-mediated condition that predominantly affects the axial skeleton. The main symptom is chronic, inflammatory lower back pain, but symptoms can also include inflammatory peripheral arthritis, enthesitis, and extra-musculoskeletal manifestations such as psoriasis, inflammatory bowel disease, and uveitis [1].
The term axSpA encompasses both ankylosing spondylitis (AS), also known as radiographic axSpA, and non-radiographic axSpA (nr-axSpA) [2]. The condition usually starts in the third decade of life with a male to female ratio of 2:1 for AS and equal gender prevalence for nr-axSpA [3]. In its advanced stage, AS leads to fusion of sacroiliac joints and the spine.
The prevalence of axSpA in the United States (US) has been estimated to be 0.9–1.4% [4], while the prevalence in the rest of the world ranges from 9 to 30 per 10,000 persons, depending on geographic area, study population, data source, or case definition. The progression of patients with nr-axSpA to AS is slow, with estimates of 5.1% in 5 years and 19% in 10 years [5]. Although patients with nr-axSpA may have shorter disease duration and lack definitive radiological changes of sacroiliitis, they demonstrate a substantial physical and social burden of illness, with self-reported disease activity and functional impairments comparable to those found in patients with AS [6].
The diagnosis of axSpA may be challenging, as no formal diagnostic criteria are available. The recent classification criteria for axSpA developed by the Assessment of Spondyloarthritis International Society (ASAS) include a combination of features such as sacroiliitis on either conventional radiography or magnetic resonance imaging (MRI), presence of human leucocyte antigen B27 (HLA-B27), raised C-reactive protein, and other associated clinical characteristics [7]. With only 37% of patients with AS in the US diagnosed by rheumatologists [8], time to diagnosis has been reported to be as long as 14 years in some patients, indicating the potential failure to recognise the condition by non-rheumatologists [9].
Delayed diagnosis is associated with more functional impairment, higher healthcare costs, and worse quality of life and work productivity outcomes in patients with AS [10, 11]. Early diagnosis results in patients receiving therapy sooner where it is considered most effective, which should lead to reduced burdens and improved outcomes [12].
The goals of treatment are to alleviate symptoms, improve functioning, maintain the ability to work, decrease disease complications, and avoid skeletal damage as much as possible [13]. Current ASAS-European League Against Rheumatism (EULAR) treatment guidelines for axSpA recommend continuous treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) as a first-choice therapy. In patients who do not improve with continuous NSAID treatment, biological disease-modifying antirheumatic drugs (bDMARDs), including tumour necrosis factor inhibitors (TNFi) and interleukin-17 (IL-17) inhibitors (e.g. secukinumab and ixekizumab), are recommended for patients with high disease activity despite the use (or intolerance/contraindication) of at least two NSAIDs [14]. Conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), such as sulfasalazine, should be considered for axSpA patients with peripheral involvement, or when a TNFi or IL-17 is not available or appropriate [14].
In the US, the 2019 guidelines from the American College of Rheumatology, Spondylitis Association of America, and the Spondyloarthritis Research and Treatment Network for the treatment of AS and nr-axSpA also recommend TNFi over an IL-17 inhibitor as the first bDMARD to be used, while secukinumab or ixekizumab is conditionally recommended over the use of a second TNFi in patients with primary nonresponse to the first TNFi [13]. Either a TNFi or an IL-17 inhibitor is favoured over the Janus kinase inhibitor, tofacitinib [13].
The diagnosis, treatment, and burden of AS have been less researched in Central Eastern European countries (CEE) than in the US [15, 16]. Given the increasing importance of these emerging markets, with recent trials recruiting a majority of patients from this region, the aim of this study was to compare the treatment journey of patients with AS, including diagnosis, treatment patterns, and burden of disease in both the CEE and the US from a real-world perspective.
Materials and methods
Study design
This study was an analysis of secondary data drawn from the Adelphi AxSpA Disease Specific Programme (DSP)™, a point-in-time survey of rheumatologists and their consulting patients in the US, Czech Republic, Poland, Russia, and Ukraine. DSP™s are multinational surveys collecting information on real-world clinical practice, designed to identify current disease management, and patient- and physician-reported disease impact [17].
Data were collated from a DSP™ which was conducted in the US between April and October 2018, and in CEE between August and November 2019. Data were collected through physician- and patient-completed record forms and included clinical information and patient-reported outcomes (PROs).
A geographically representative sample of physicians were recruited to participate in the DSP™ and gave informed consent to participate, with physicians recruited by local field-based interviewers using publicly available lists and screened against eligibility criteria. The data collection setting was secondary care rheumatology services (public or private hospitals, clinics, or offices). While minimal inclusion criteria governed the selection, participation was influenced by willingness to complete the survey. Following screening, physicians included in the survey were invited to complete a pre-specified patient record form (PRF) questionnaire for 3–6 consecutive patients with AS who visited for routine care. PRFs included detailed questions on patient demographics, clinical assessments, medication use, and treatment history. Patients were eligible for inclusion if aged 18 years or older, with a physician-confirmed diagnosis of AS. There were no restrictions according to treatments, clinical features such as disease activity/severity or demographics. Each patient with a physician-completed questionnaire was invited to voluntarily fill out a patient self-completed (PSC) questionnaire after providing informed consent. Patients completed their questionnaires independently from physicians, returning them in sealed envelopes to ensure confidentiality.
Data captured included physicians’ approach to AS diagnosis, tests and assessments used to confirm diagnosis, patient demographic and clinical characteristics (including the proportion of patients who were known to be HLA-B27 positive), the duration from initial symptoms to an AS diagnosis, fulfilment of classification criteria, treatment patterns (including the use of advanced treatment by AS disease activity), and patient-reported burden of disease from AS, using validated measures. Patients were invited to voluntarily complete the following validated measures of disease activity, quality of life (QoL), and general health status and productivity: the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) [18], the Ankylosing Spondylitis Quality of Life Questionnaire (ASQoL) [19], the EuroQoL 5D (EQ-5D) [20], Work Productivity and Activity Impairment (WPAI) questionnaires [21], and the ASAS Health Index (ASAS HI) [22].
Data analysis
All PRFs were completed online to minimise the issue of missing data. If physicians did not know or have access to historical medical records, there was the option to select “don’t know” or “not applicable.” Missing data were not imputed; therefore, the base of patient data for analysis could vary from variable to variable and is reported separately for each analysis, thereby enabling the calculation of the number of missing patients. Patients were grouped according to geographical area of origin (US or CEE) and each variable of interest was reported and compared across geographical area (US vs CEE) using methods appropriate to variable type.
Physician-level data from the physician survey, patient demographics, and underlying patient condition (including age, gender, body mass index [BMI], comorbid conditions, and symptoms) from PRFs were compared using Fisher’s exact tests (for binary categorical variables) [23]. For categorical variables with more than two categories, the Fisher-Freeman-Halton test with the Mehta and Patel extension of the Fisher’s exact test was used [24, 25].
For outcomes (see Online Resource 1 for a variables list), a multivariable regression approach was used to adjust for potential confounding from age, gender, BMI, and comorbidities (using the Charlson Comorbidity Index [CCI]). In each case, a binary variable was included that represented region (US/CEE), together with the confounding variables.
For continuous outcomes, a generalized linear model with normal distribution and an identity link function was employed [26]. For categorical outcomes, a logistic regression (for the binary case) or multinomial logistic regression (for the case of more than 2 groups) was employed [27]. The output for each fitted model included means (least squares) and the p-values associated with CEE (compared with the US as the reference country).
For time-to-event outcomes, t-tests were performed. Where statistical tests were performed, p-values < 0.05 were considered statistically significant. Standard errors were adjusted for potential clustering within physicians [28].
All analyses used Stata Statistical Software: Release 15 (StataCorp LP, College Station, TX).
A sensitivity analysis was also conducted, which replicated the primary analysis but selected patients who were reported by physicians to have sacroiliitis identified by x-ray at diagnosis. In a real-world population of AS patients, not all patients were able to be confirmed as having sacroiliitis identified by x-ray at diagnosis. The purpose of conducting a sensitivity analysis was to, as closely as possible, match the AS population in clinical trials and also allow comment on the applicability of the results in patients with sacroiliitis identified by x-ray at diagnosis to the entire sample (see Online Resources 1–7).
Ethical considerations
The DSP™ complies with all relevant market research guidelines and legal obligations. Data were collected according to European Pharmaceutical Marketing Research Association guidelines and thus did not require ethics committee approvals [29]. Namely, the DSP™ is non-interventional and employs solely retrospective data collection, and no identifiable protected health information was extracted during the course of the study.
Results
Study population
The sample included a total of 487 patients recruited from 88 rheumatologists in the US and 922 patients from 126 rheumatologists in CEE with a physician-confirmed diagnosis of AS. Patient-reported data were collected for 55% of US patients and 86% of CEE patients (US 296, CEE 793).
Patient demographic and clinical characteristics
Key patient demographics and disease characteristics are summarised in Table 1. Patient characteristics did not differ significantly between the geographical regions in terms of age and sex, and the majority of patients were white/Caucasian in both populations. In CEE, the proportion of patients currently employed was lower than in the US, with a significantly higher proportion being smokers or ex-smokers, and those in CEE also had a lower mean BMI. Physician-reported severity of condition was similar in both regions, with over half of patients being categorised as moderate. The proportion of patients with sacroiliitis on x-ray was significantly higher in CEE relative to the US, while the key disease features of inflammatory back pain, morning stiffness for more than 30 min, enthesitis, and dactylitis at diagnosis were similar in both countries (Table 1).
Duration from initial symptoms to an AS diagnosis
The time from onset of symptoms to first consultation and final diagnosis of AS was significantly longer in CEE than in the US (Table 2). Patients in both regions were mainly referred by their family practitioner or other specialist, and a diagnosis was almost always made by a rheumatologist. However, the mean duration until referral to the current rheumatologist was 13.0 months in CEE compared with 4.8 months in the US (Table 3). In both the US and CEE, reasons for a delay in diagnosis included awaiting referral to the correct healthcare professional and awaiting tests to confirm diagnosis (Table 3). In CEE, one-third of patients experienced a delay due to initial diagnosis of another condition, significantly more than in the US. Patients in both the US and CEE waited a similar length of time from start of symptoms before seeking medical advice, most commonly reporting that they waited to see if their symptoms would resolve spontaneously (Table 3). Patients in the US were significantly more concerned about the cost of treatment (Table 3) but received a bDMARD sooner than patients in CEE (mean: 2.7 vs 4.0 years after initial diagnosis, p = 0.004) (Table 3).
Treatment patterns
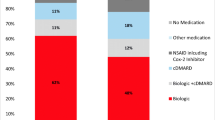
At diagnosis, a higher use of csDMARDs and injected steroids was reported in CEE compared with the US. Almost a quarter of patients in the US received a bDMARD at diagnosis, compared to 10% in CEE (Table 4). At the time of the current study, almost three-quarters of patients in the US were receiving a bDMARD, with levels of bDMARD use in CEE significantly lower than the US (Table 4), although the mean number of bDMARD treatments per patient was similar in both the US and CEE (mean [SD] 1.3 [0.6] and 1.2 [0.6], respectively). The number of csDMARDs initiated before a bDMARD was similar in both the US and CEE (mean [SD] 1.3 [0.6] and 1.2 [0.4], respectively), with sulfasalazine being prescribed in significantly more patients in CEE than the US (79.3% and 49.4%, respectively; p < 0.001). Methotrexate was preferred over sulfasalazine in 62.7% of patients in the US, but in only 50.6% of patients in CEE.
Patient-reported outcomes
Disease activity (BASDAI) was greater in patients in CEE vs US (4.2 vs 3.1, respectively, p < 0.05). In the US, 68.8% of patients had a BASDAI score of 4 or less, while in CEE, it was 48.2% (p < 0.05). Patients in the US had a lower score (indicating better QoL) on the ASQoL than those in CEE (6.2 vs 8.4, respectively, p < 0.05). In the US, 65.0% of patients had an ASQoL score of 8 or less, while in CEE, it was 50.7% (p < 0.05). A significantly lower mean EQ-5D index for the CEE population was observed relative to the US (0.7 vs 0.8, respectively, p < 0.05). The mean ASAS HI score for the US population indicated a moderate impairment in functioning, while patients in CEE had significantly greater and more severe impairment (5.7 vs 8.2, respectively, p < 0.05). In the US, 39.3% of patients had a score of less than 4; 31.4% had a score of 4–8; and 29.3% had a score greater than 8 (p < 0.05). In CEE, these proportions were 21.0%, 29.9%, and 49.1%, respectively (p < 0.05). Patients in CEE also had a significantly higher mean pain score than those in the US (4.3 vs 3.5, respectively, p < 0.05) (means in Fig. 1, proportions not shown). Impairment in work productivity was significantly higher among patients in CEE than in the US (33% vs 23%, respectively, p < 0.05). Activity impairment was also significantly higher in CEE than in the US (41% vs 30%, respectively, p < 0.05) (Fig. 2).
ASQoL, ASAS HI, EQ-5D, and BASDAI mean scores. Least square means and p-values are from an ordinary least squares regression with additional covariates: age, sex, BMI, and Charlson Comorbidity Index. *p-value CEE vs US < 0.05. Abbreviations: ASAS HI, Assessment of Spondyloarthritis International Society Health Index; ASQoL, Ankylosing Spondylitis Quality of Life Questionnaire; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BMI, body mass index; CEE, Central Eastern European countries; EQ-5D, EuroQoL 5D; QoL, quality of life; SE, standard error; US, United States
Sensitivity analysis
A sensitivity analysis that replicated the primary analysis found that the results in patients who were confirmed to have sacroiliitis on x-ray at diagnosis (US n = 272, 56.7%; CEE n = 625, 68.4%) were highly consistent with the overall sample results in terms of demographics, clinical status, treatments prescribed, journey to patient diagnosis, and patient-reported outcomes (see Online Resources 2–7). Minor differences included physician-perceived severity at diagnosis and prevalence of a positive HLA-B27 result, with patients in the sensitivity analysis more frequently being perceived as “severe” (overall analysis—US: 35.8% and CEE: 38.6% severe; sensitivity analysis—US: 40.3% and CEE: 42.5% severe), and more frequently being positive for HLA-B27 (overall analysis—US: 67.6% and CEE: 67.8% positive; sensitivity analysis—US: 77.2% and CEE: 74.7% positive). Treatment patterns between the two analyses were also consistent, with minor difference including more bDMARD prescribed at both diagnosis and currently in the sensitivity analysis population (overall analysis bDMARD at diagnosis—US: 22.9% and CEE: 10.2%; sensitivity analysis bDMARD at diagnosis—US: 30.1% and CEE: 12.2%; overall analysis bDMARD currently—US: 71.0% and CEE: 27.9%; sensitivity analysis bDMARD currently—US: 75.9% and CEE: 31.9%).
Discussion
Relatively few studies have compared clinical outcomes in patients with AS from different geographical regions [15, 16] and this study of patients with AS is novel in comparing diagnostic procedures, treatment patterns, and outcomes in the US and CEE from a real-world perspective. These regions were selected in large part due to their heterogeneity and their importance as emerging markets. Another factor was the ability to recruit an optimal number of physicians and patients to establish a robust dataset for the analyses. This will allow for a more thorough interpretation of trial results, given that recent trials for AS and nr-axSpA are recruiting most patients from the CEE region. We also wanted to understand whether any similarities or differences in these populations could lead to any differences in treatment effect across regions, given new drug approvals typically occur in the US first.
Overall, the study shows that treatment patterns are very different, with reported higher use of csDMARDs and injected corticosteroids at diagnosis, and lower levels of bDMARD use reported at both diagnosis and at the current consultation in the CEE than in the US, potentially indicating a lack of access to treatment and different approach to care in the CEE region.
It is well documented that patients with AS can experience delays in diagnosis [10], and evidence from studies across the world indicates that diagnostic delay may extend over several years [8, 30]. In the CEE, the referral time from the initial healthcare professional to rheumatologist was significantly longer than in the US, suggesting that the primary care system in CEE is a potential source of delays. The time to diagnosis was also found to take longer in CEE than in the US. Also, patients in CEE reported poorer QoL, greater work and activity impairment, more work time missed, and higher disease activity than patients in the US.
Two-thirds of patients in both the US and CEE were reported to be HLA-B27 positive, which is lower than other studies that have suggested the prevalence of HLA-B27 in patients with AS to be as high as 80–94% [31,32,33]. Since the data collected in this real-world study employed a non-interventional approach, data was only available if a positive result was recorded in medical records, we were not able to ascertain if more patients were tested and proved HLA-B27 negative or were untested. It is feasible that patients in this sample had not been tested for HLA-B27 during their routine care, leading to underreporting or underrepresenting of positive HLA-B27 status.
After failure of NSAIDs, biologic DMARDs are the only proven efficacious therapies for the treatment of axSpA, with the suppression of inflammation shown to improve and maintain QoL in patients [34]. However, due to the high cost of biologics, considerable differences in their utilization exist, with many countries restricting access despite professional society guideline recommendations. The adoption of biologics by healthcare providers has been reported to be less in many CEE countries [35, 36], and differences in utilization have been reported across medical specialties, healthcare providers, and at a regional and national level [37].
In our real-world study, over three-quarters of patients (72.1%) with AS in the US received bDMARDs compared with approximately one-quarter of patients (27.3%) in CEE. It has been previously reported that access to such medication may be limited in CEE countries due to reimbursement systems only covering low cost treatments, with a lack of access to rheumatological care in particular [38].
In terms of PROs, we found that the mean BASDAI was higher in patients in CEE (mean score 4.2 vs 3.1 in US). The patient-acceptable symptom state (PASS) is estimated as a score of 4.1 [39], which a significantly greater proportion of patients in the US had achieved (68.8% vs 48.2% in CEE). Patients in the US vs CEE also had a significantly lower mean ASQoL score (6.2 vs 8.4). The PASS for ASQoL has been calculated at < 8.0 [40], which a significantly greater proportion of patients in the US had achieved (65.0% vs 50.7% in CEE), indicating a higher proportion of patients experience an acceptable quality of life in the US. The mean EQ-5D index for patients in the US was similar to the reported population norm of 0.81 for the age group 45–54 [41], while a significantly lower index for the CEE population was observed indicating a poorer QoL in CEE patients in this sample. Scores of over 8 on ASAS HI are considered to represent severe disease [42, 43]. In this study, less than one-third of US patients had severe disease, whereas in CEE, half of all patients had severe disease.
Health-related work productivity is generally described in terms of absenteeism (time away from work), presenteeism (extent to which work productivity is impaired while at work), and disability (limit to activities). Previous studies examining the impact of chronic conditions on productivity have estimated that on the job work impairment ranged from a 18–36% decrement in ability to function at work [44]. This study showed comparable results. In terms of disability (activity impairment), this was found to be significantly higher in CEE than the US. Absenteeism was double that of the US in CEE.
Limitations
This was a non-interventional study, with physicians completing forms on consecutively consulting patients with AS. However, selection bias was possible owing to the fact that physicians surveyed represented a convenience sample and may not be representative of the overall population of physicians treating patients with AS in the US and CEE. Eligible patients were screened and selected by physicians, and it is therefore recognised that patients who were visiting physicians more often were more likely to have been included in the study. Nonetheless, this is reflective of real-world clinical practice, and representative of a consulting population. To minimise these factors, physicians were recruited from a diverse geographical spread and mixed private/public practice. The selection of patients was made based on physicians’ clinical judgement; they might have not always used a radiograph to confirm sacroiliitis at time of diagnosis or completion of the forms or they may have done a radiograph at a different time point. Participating patients were encouraged, but not mandated, to complete all questionnaires, such that base sizes fluctuated across different variables. Finally, it is acknowledged that the study relied on the accuracy of physicians when completing each PRF. To minimise the risk of collecting inaccurate data, PRFs were relatively short and user-friendly with electronic routing and logic applied to ensure no contradictions in responses and, where appropriate, physicians were provided the opportunity of entering “don’t know” if the information was not available.
Conclusion
This study was novel in providing data on the real-world approach to the diagnosis, treatment, and burden of axSpA and, specifically, AS, in CEE compared with the US. The reported delay in diagnosis and poorer access to biologics may have resulted in the higher disease activity, greater levels of pain, and poorer outcomes as reported by patients with axSpA in CEE. Providing early access to treatment with bDMARDs may improve symptoms and QoL, and increase work productivity and daily activities in patients with AS in CEE countries.
Data availability
All data that support the findings of this study are the intellectual property of Adelphi Real World. All requests for access should be addressed directly to E. Holdsworth at elizabeth.holdsworth@adelphigroup.com.
Code availability
All analyses carried out in the course of this study used Stata Statistical Software: Release 15 (StataCorp LP, College Station, TX). Further inquiries regarding details of data analysis should be addressed to E. Holdsworth at elizabeth.holdsworth@adelphigroup.com.
Change history
27 January 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10067-022-06069-3
References
Erol K, Gok K, Cengiz G et al (2018) Extra-articular manifestations and burden of disease in patients with radiographic and non-radiographic axial spondyloarthritis. Acta Reumatol Port 43(1):32–39
Wright GC, Kaine J, Deodhar A (2020) Understanding differences between men and women with axial spondyloarthritis. Semin Arthritis Rheum 50(4):687–694
Sieper J, Poddubnyy D (2017) Axial spondyloarthritis. Lancet 390(10089):73–84
Reveille JD, Witter JP, Weisman MH (2012) Prevalence of axial spondylarthritis in the United States: estimates from a cross-sectional survey. Arthritis Care Res (Hoboken) 64(6):905–910
Wang R, Ward MM (2018) Epidemiology of axial spondyloarthritis: an update. Curr Opin Rheumatol 30(2):137–143
Boonen A, Sieper J, van der Heijde D et al (2015) The burden of non-radiographic axial spondyloarthritis. Semin Arthritis Rheum 44(5):556–562
Rudwaleit M, van der Heijde D, Landewe R et al (2009) The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 68(6):777–783
Danve A, Deodhar A (2019) Axial spondyloarthritis in the USA: diagnostic challenges and missed opportunities. Clin Rheumatol 38(3):625–634
Deodhar A, Mease PJ, Reveille JD et al (2016) Frequency of axial spondyloarthritis diagnosis among patients seen by US rheumatologists for evaluation of chronic back pain. Arthritis Rheumatol 68(7):1669–1676
Seo MR, Baek HL, Yoon HH et al (2015) Delayed diagnosis is linked to worse outcomes and unfavourable treatment responses in patients with axial spondyloarthritis. Clin Rheumatol 34(8):1397–1405
Garrido-Cumbrera M, Poddubnyy D, Gossec L et al (2019) The European map of axial spondyloarthritis: capturing the patient perspective-an analysis of 2846 patients across 13 countries. Curr Rheumatol Rep 21(5):19
Yi E, Ahuja A, Rajput T et al (2020) Clinical, economic, and humanistic burden associated with delayed diagnosis of axial spondyloarthritis: a systematic review. Rheumatol Ther 7(1):65–87
Ward MM, Deodhar A, Gensler LS et al (2019) 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Care Res (Hoboken) 71(10):1285–1299
van der Heijde D, Ramiro S, Landewé R et al (2017) 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 76(6):978–991
Opris-Belinski D, Erdes SF, Grazio S et al (2018) Impact of adalimumab on clinical outcomes, healthcare resource utilization, and sick leave in patients with ankylosing spondylitis: an observational study from five Central and Eastern European countries. Drugs Context 7:212556
Torre-Alonso JC, Queiro R, Comellas M et al (2018) Patient-reported outcomes in European spondyloarthritis patients: a systematic review of the literature. Patient Prefer Adherence 12:733–747
Anderson P, Benford M, Harris N et al (2008) Real-world physician and patient behaviour across countries: Disease-Specific Programmes - a means to understand. Curr Med Res Opin 24(11):3063–3072
Garrett S, Jenkinson T, Kennedy LG et al (1994) A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 21(12):2286–2291
Packham JC, Jordan KP, Haywood KL et al (2012) Evaluation of Ankylosing Spondylitis Quality of Life Questionnaire: responsiveness of a new patient-reported outcome measure. Rheumatology (Oxford) 51(4):707–714
Whynes DK (2008) Correspondence between EQ-5D health state classifications and EQ VAS scores. Health Qual Life Outcomes 7(6):94
Reilly MC, Zbrozek AS, Dukes EM (1993) The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 4(5):353–365
Kiltz U, van der Heijde D, Boonen A et al (2018) Measurement properties of the ASAS Health Index: results of a global study in patients with axial and peripheral spondyloarthritis. Ann Rheum Dis 77(9):1311–1317
Fisher RA (1934) Statistical methods for research workers, 5th edn. Oliver & Boyd, Edinburgh
Freeman GH, Halton JH (1951) Note on an exact treatment of contingency, goodness of fit and other problems of significance. Biometrika 38(1–2):141–149
Mehta CR, Patel NR (1983) A network algorithm for performing Fisher’s exact test in rxc contingecy tables. Journal of the American Statistical Associaition 78:427–434
McCullagh P, Nelder JA (1989) Generalized linear models: monographs on statistics and applied probability 37, 2nd edn. Chapman and Hall, London
Agresti A (2012) Categorical data analysis, 3rd edn. John Wiley & Sons, Hoboken
Rogers WH (1994) Regression standard errors in clustered samples. Stata Technical Bulletin 13(3):19–23
EphRMA (2020) European Pharmaceutical Market Research Association (EphMRA) code of conduct 2020. Available at https://www.ephmra.org/standards/code-of-conduct-aer/
Sørensen J, Hetland ML (2015) Diagnostic delay in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis: results from the Danish nationwide DANBIO registry. Ann Rheum Dis 74(3):e12
Arévalo M, Gratacós Masmitjà J, Moreno M et al (2018) Influence of HLA-B27 on the ankylosing spondylitis phenotype: results from the REGISPONSER database. Arthritis Res Ther 20(1):221
de Winter JJ, van Mens LJ, van der Heijde D et al (2016) Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis Res Ther 18(1):196
Fernández-Sueiro JL, Alonso C, Blanco FJ et al (2004) Prevalence of HLA-B27 and subtypes of HLA-B27 associated with ankylosing spondylitis in Galicia. Spain. Clin Exp Rheumatol 22(4):465–8
Rohde G, Berg KH, Pripp AH et al (2020) No deterioration in health-related quality of life in patients with axial spondyloarthritis followed for 5 years in ordinary outpatient clinics in the biological treatment era. Qual Life Res 29(1):99–107
Orlewska E, Ancuta I, Anic B et al (2011) Access to biologic treatment for rheumatoid arthritis in Central and Eastern European (CEE) countries. Med Sci Monit 17(4):Sr1-13
Péntek M, Poór G, Wiland P et al (2014) Biological therapy in inflammatory rheumatic diseases: issues in Central and Eastern European countries. Eur J Health Econ 15(Suppl 1):S35-43
Baumgart DC, Misery L, Naeyaert S et al (2019) Biological therapies in immune-mediated inflammatory diseases: can biosimilars reduce access inequities? Front Pharmacol 10:279
Nasonov EL, Karateev DE (2015) Does Russia need a treat-to-target initiative? Rheumatology (Oxford) 54(3):381–382
Kviatkovsky MJ, Ramiro S, Landewé R et al (2016) The minimum clinically important improvement and patient-acceptable symptom state in the BASDAI and BASFI for patients with ankylosing spondylitis. J Rheumatol 43(9):1680–1686
Maksymowych WP, Richardson R, Mallon C et al (2007) Evaluation and validation of the patient acceptable symptom state (PASS) in patients with ankylosing spondylitis. Arthritis Rheum 57(1):133–139
Szende A, Janssen B, Cabases J (eds) (2014) Self-reported population health: an international perspective based on EQ-5D. Springer, Dordrecht
Di Carlo M, Becciolini A, Lato V et al (2017) The 12-item Psoriatic Arthritis Impact of Disease Questionnaire: construct validity, reliability, and interpretability in a clinical setting. J Rheumatol 44(3):279–285
Di Carlo M, Lato V, Carotti M et al (2016) Clinimetric properties of the ASAS Health Index in a cohort of Italian patients with axial spondyloarthritis. Health Qual Life Outcomes 17(14):78
Collins JJ, Baase CM, Sharda CE et al (2005) The assessment of chronic health conditions on work performance, absence, and total economic impact for employers. J Occup Environ Med 47(6):547–557
Acknowledgements
Medical writing support under the guidance of the authors was provided by K. Ian Johnson, a paid sub-contractor of Adelphi Real World which received funding from Pfizer in connection with the development of this manuscript.
Funding
Data collection was undertaken by Adelphi Real World as part of a survey, entitled the Adelphi AxSpA Disease Specific Programme™, sponsored by multiple pharmaceutical companies of which one was Pfizer Inc. Pfizer Inc. did not influence the original survey through either contribution to the design of questionnaires or data collection. The study described here using data from the Adelphi AxSpA Disease Specific Programme™ was funded and sponsored by Pfizer Inc.
Author information
Authors and Affiliations
Contributions
All authors were involved in (1) conception or design, or analysis and interpretation of data; (2) drafting and revising the article; (3) providing intellectual content of critical importance to the work described; and (4) final approval of the version to be published, and therefore meet the criteria for authorship in accordance with the International Committee of Medical Journal Editors guidelines. In addition, all named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Corresponding author
Ethics declarations
Ethics approval
Not applicable. The DSP™ complies with all relevant market research guidelines and legal obligations. Data were collected according to European Pharmaceutical Marketing Research Association guidelines and thus did not require ethics committee approvals.
Consent to participate
Using a check box, patients provided informed consent for use of their anonymized and aggregated data for research and publication in scientific journals. Data were collected in such a way that patients and physicians could not be identified directly; all data were aggregated and de-identified before receipt by Adelphi Real World.
Conflict of interest
TK received research grants from Pfizer, and consultancy/speaking fees from Janssen, Pfizer, MCD, UCB, Novartis, Abbvie, Biocad, Lilly and Novartis-Sandoz.
OD, LF, LW, RV, and JCC are employees of Pfizer Inc. and own stocks in Pfizer Inc.
EH, GM, and SM are employees of Adelphi Real World. Adelphi Real World received funding from Pfizer Inc. for this analysis and manuscript development.
AD received research grants and consultancy/speaking fees from Pfizer Inc.; he has participated in advisory boards for Pfizer Inc.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Korotaeva, T., Dina, O., Holdsworth, E. et al. Investigating diagnosis, treatment, and burden of disease in patients with ankylosing spondylitis in Central Eastern Europe and the United States: a real-world study. Clin Rheumatol 40, 4915–4926 (2021). https://doi.org/10.1007/s10067-021-05864-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-021-05864-8