Abstract
Introduction
Biologics effectively manage symptoms and disease activity in rheumatoid arthritis (RA), but their long-term effects remain unclear.
Method
Longitudinal data were examined from the Brigham and Women’s Rheumatoid Arthritis Sequential Study (BRASS) registry. Linear regression modeled the effect of biologic exposure on changes in disease activity (Disease Activity Score-28 with C-reactive protein [DAS28-CRP]), functional status (modified Health Assessment Questionnaire [mHAQ]), and RA severity (Routine Assessment of Patient Index Data [RAPID3]). Biologic exposure was the ratio of time on a biologic relative to time participating in the BRASS cohort.
Results
The analysis included 1395 RA patients, 82.3% female, with 6783 unique study visits from 2003 to 2015. At the patient’s first visit, mean (SD) age was 56.3 (14.2) years and mean (SD) duration of RA was 12.7 (11.9) years. Average follow-up duration was 5.59 years (range, 1–13). Over time, DAS28-CRP, mHAQ, and RAPID3 scores decreased as the biologic exposure ratio increased. In repeated measures regression models, increased biologic exposure was significantly associated with decreased DAS28-CRP score (β = − 0.647; P < 0.001), decreased mHAQ score (β = − 0.096; P < 0.001), and decreased RAPID3 score (β = − 0.724; P < 0.001) during follow-up. Methotrexate use at baseline predicted decreased DAS28-CRP, mHAQ, and RAPID3 scores during follow-up. Biologic use at baseline predicted increased DAS28-CRP or mHAQ during follow-up.
Conclusions
Increased biologic exposure is associated with decreased disease activity, function impairment, and RA severity. Future studies should examine whether earlier initiation of biologics improves patient outcomes in RA.
Trial Registration
ClinicalTrials.gov, NCT01793103
Key Points • Biologics effectively manage symptoms and disease activity in rheumatoid arthritis (RA), but their long-term effects remain unclear. • In this analysis of longitudinal annual population samples of 1395 RA patients in the Brigham and Women’s Rheumatoid Arthritis Sequential Study (BRASS) registry, disease activity, function, and severity scores improved as time on biologic therapy increased. • In repeated measures regression models, time on biologic therapy was a significant predictor of improved outcomes for disease activity, function, and RA severity. • Further studies should examine whether earlier initiation of biologics limits the long-term effect of inflammation on RA outcomes. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biologics have become the standard of care for treating moderate to severe rheumatoid arthritis (RA) in patients with an inadequate response to small molecule disease-modifying antirheumatic drugs (DMARDs) [1,2,3]. Although biologics have been proven effective in managing RA symptoms and disease activity across numerous clinical studies [4], their long-term effect on disease activity and patient function remains unclear. A national registry study in Sweden and a real-world prospective study of 4651 adults with RA in the USA both reported reduced disability due to RA after biologic treatment options were introduced in 1998, compared with the period before biologics were available [5, 6].
Control of inflammatory processes is known to limit joint damage and disease progression. It stands to reason that use of biologic DMARDs, especially early in disease, could help to reduce the overall severity of disease within the RA population [7,8,9]. Combining biologic therapy with nonbiologic DMARD therapy early in patients with recent onset of RA reduces joint damage and disability compared with sequential or “step-up” combination therapy with nonbiologic DMARDs followed by biologic therapy [7,8,9]. When tumor necrosis factor inhibitors became available for the treatment of RA, patients often initiated biologic therapy later in the treatment course, when disability was already more severe [10]. Later initiation of biologic therapy in patients with established RA has a similar effect on joint damage, but it may reduce disability less effectively [7, 11].
Treatment guidelines for RA have evolved over time to support earlier and more aggressive use of biologics [1, 12, 13]. Older guidelines recommended initiating biologic therapy in patients with early RA (< 6 months) only for patients with features of poor prognosis [12, 13]. Based on the available low-to-moderate evidence, current guidelines recommend either adding biologic therapy or combining nonbiologic DMARDs in a patient whose disease activity remains moderate or high despite therapy with a small molecule DMARD, regardless of whether the patient has early RA or established RA [1]. The optimal time to start biologic therapy in RA remains unclear, but there is some evidence that starting biologic therapy earlier is associated with greater chance of achieving low disease activity [14].
Many studies of disease burden in RA patients are limited to 1 year or less in duration. Registry study data can be used to examine whether longer-term exposure to a biologic maintains the benefits for reduced disease activity and function impairment. Previous analyses of registry data have shown that RA patients who respond to biologic therapy in the first 6–12 months usually experience persistent benefits if they continue biologic therapy for several years [15,16,17,18,19,20,21,22,23,24]. The objective of this analysis was to examine the effect of cumulative exposure to biologics on patient outcomes over time, as well as predictors for improvement in outcomes.
Materials and methods
Data source
The Brigham and Women’s Rheumatoid Arthritis Sequential Study (BRASS) registry includes clinical and patient-reported outcome data from a cohort of more than 1400 RA patients from a single academic medical center at varying stages of disease progression. BRASS is an observational and longitudinal registry that follows patients over a period of 15 years.
The registry was established in March 2003 at the Robert Breck Brigham Arthritis Center in Boston, MA. Approximately 2700 variables are collected as part of the registry, including patient-reported data, physician assessments, and laboratory tests. Patients complete surveys to record patient-reported outcomes twice annually, while physician assessments and laboratory tests are performed at annual study visits.
Patients have entered the registry over time, with significant enrollment in the early years of the registry and in the early 2010s. Individual patients have contributed from 1 to 13 years of data depending, in part, on timing of enrollment, with an average of 5.59 years of participation. Years in which patients contributed data were not necessarily continuous. For example, a patient contributing 5 years of data could have provided survey data in 5 noncontinuous years of the registry. The BRASS registry has produced a large prospective database; analyses of the BRASS dataset have yielded insight into diagnosis, biomarkers, treatment regimens, and patient outcomes for the RA patient population [25,26,27,28,29,30,31].
Study procedures and written informed consent were approved by the Institutional Review Board of Brigham and Women’s Hospital, Partners Health Care, Boston, MA. A copy of the protocol and the informed consent form for enrollment in the BRASS registry were submitted to the Institutional Review Board of Brigham and Women’s Hospital, and written approval was obtained.
Biologic utilization
Any exposure to a given medication or class of medications for RA in a calendar year was determined from the twice-annual patient self-report surveys. The biologic exposure ratio was calculated at each time point as the ratio of the patient’s total time on biologics (from the patient self-report surveys), divided by their total time in the BRASS registry, which resulted in a continuous proportion for each patient that ranged from 0 (no biologic use) to 1 (continuous biologic use). Because the biologic exposure ratio was calculated at every time point, an individual’s biologic exposure could vary over the course of the study.
Outcomes
Three outcomes were assessed in this analysis to understand clinical and functional status and disease severity: the Disease Activity Score-28 with C-reactive protein (DAS28-CRP), the modified Health Assessment Questionnaire (mHAQ), and the Routine Assessment of Patient Index Data (RAPID3). All outcome data were derived from the annual study visit.
The DAS28 is a clinical composite score derived from patient-reported data, physician assessments, and laboratory tests that provide an assessment of disease activity [32]. The BRASS registry includes the DAS28-CRP, which is composed of tender and swollen joint counts (28 joints assessed), a C-reactive protein laboratory value, and a patient’s global assessment score for disease activity, using a visual analog scale. Greater DAS28-CRP scores indicate greater disease activity, with cutoffs for moderate and high severity at 3.2 and 5.1, respectively [33].
The BRASS registry also includes the mHAQ, a patient-reported questionnaire that monitors functional status in patients with rheumatic diseases [34]. The questionnaire includes eight activities of daily living (dressing; getting in/out of bed; lifting a full cup/glass; walking on flat ground; washing/drying entire body; bending down to pick up clothing off the floor; turning taps on/off; and getting in/out of a bus, car, train, or airplane). Scores range from 0 to 3, with lower scores indicating a better functional status.
The RAPID3 is another patient-reported scale in the BRASS registry that provides a quick assessment of RA severity [35]. It includes a subset of variables within the multi-dimensional Health Assessment Questionnaire, a patient global assessment for pain, and a patient global assessment for overall health. Scores range from 0 to 10, with cutoffs for remission (0.0–1.0), low disease activity (> 1.0–2.0), moderate disease activity (> 2.0–4.0), and high disease activity (> 4.0).
Statistical methods
Descriptive statistics (mean and standard deviation [SD] for continuous variables; frequencies and percentages for categorical variables) were calculated for patient demographics. Baseline values were derived from the patient’s first annual study visit in the BRASS registry.
The effect of the biologic exposure ratio on RA patient outcomes was assessed using linear mixed effects repeated measures models with binary and categorical variables treated as classes. These models controlled for the correlations among repeated measures on the same individual and were not affected by random missing data. Covariates included in initial models were to control for various patient demographic and treatment factors known to be clinically relevant to RA outcomes (Table 1). Model development began with analysis of clinically relevant covariates and culminated with the best fit model; clinically relevant covariates were determined by the study team, which included practicing rheumatologists. Covariates included in the final best fit model for each outcome were determined through an iterative process that considered overfitting and collinearity, among other factors. The best fit model was identified based on model fit statistics, significance of individual covariates, and the contribution of individual covariates to model fit as indicated by the t-value. Model assumptions were checked with both distribution analyses of the raw data and the residuals of the model. Model residuals were normally distributed, supporting use of linear mixed effects repeated measures models without transformation of the data. Only variables included in the best fit models are presented.
Three sets of Pearson zero-order correlation coefficients (among outcome variables, among covariates, and between covariates and outcomes) were performed for each year of the study to examine possible changes in variable relationships over absolute time. Covariates that correlated with outcomes with a p ≤ 0.05 and had an r ≥ 0.10 for at least 1 year of the study were included in the preliminary full models; all covariates met these criteria. Correlation coefficients among covariates with an r ≥ 0.90 were considered to have potentially high levels of collinearity. Further analyses of collinearity among covariates and interaction terms were conducted as part of model construction. All variables included in the best fit models had a variance inflation index < 5 or a condition index < 30 and thus had low levels of collinearity.
Patient covariates and outcomes were treated as the between-subject effects and calendar year as the within-subject effect. The restricted maximum likelihood (REML) estimation method was used for all iterations. Alternative covariance structures and degrees of freedom methods were evaluated using Akaike’s index criterion (AIC), the restricted likelihood ratio test, and the Bayes index criterion (BIC). All best fit models used the between-within degrees of freedom method. The contribution of individual covariates to model fit was evaluated using the p value and t-value for individual covariates and the overall model fit statistics to determine the best fit model. Variables with a p value > 0.25 were retained if they contributed substantially to model fit, which was defined as reducing the − 2 residual log likelihood or AIC by more than 10 or the BIC by more than 2. Biologic exposure ratio was retained in the models regardless of p value.
Within-subject effects:
-
Between-subject effects:
- i :
-
1... ... .. N individuals
- j :
-
1... ... .. ni observations f or individual i
- Y ij :
-
Value of outcome measure at observation j for individual i
- Time ij :
-
Year of study at observation j for idividual i
- BER ij :
-
Value of biologic exposure ratio at time j for individual i
- β 0i :
-
Subject-specific intercept for individual i
- β 1i :
-
Subject-specific slope for variable Time for individual i
- β 2i :
-
Subject-specific slope for variable BER for individual i
- β 3i :
-
Subject-specific slope for Time by BER interaction for individual i
- γ 00 :
-
The mean intercept across all individuals
- γ 10 :
-
The mean slope across all individuals for the variable Time
- γ 20 :
-
The mean slope across all individuals for the variable BER
- γ 30 :
-
The mean slope across all individuals for the Time by BER interaction
- Covariate i :
-
Baseline covariate for individual i
- γ 01 :
-
Slope of between-subjects effects equation predicting β0ifrom Covariatei; indicates systematic deviation from γ00resulting from Covariatei
- γ 11 :
-
Slope of between-subjects effects equation predicting β1ifrom Covariatei; indicates systematic deviation from γ10resulting from Covariatei
- γ 21 :
-
Slope of between-subjects effects equation predicting β2ifrom Covariatei; indicates systematic deviation from γ20resulting from Covariatei
- γ 31 :
-
Slope of between-subjects effects equation predicting β3ifrom Covariatei; indicates systematic deviation from γ30resulting from Covariatei
- U 0i :
-
Residual indicating unexplained deviation from γ 00 for individual i
- U 1i :
-
Residual indicating unexplained deviation from γ 10 for individual i
- U 2i :
-
Residual indicating unexplained deviation from γ 20 for individual i
- U 3i :
-
Residual indicating unexplained deviation from γ 30 for individual i
- ε ij :
-
Within-subjects random error
Baseline covariates included gender and highest level of education, continuous values for mHAQ and DAS28-CRP, and dichotomous indicators for any biologic use prior to enrollment (yes or no) and biologic use at baseline (yes or no). Candidate variables for time-varying covariates included for each study year were age in years, year of study, disease duration in years, binary indicators for any biologic use (yes or no) or any methotrexate use (yes or no), and smoking status (never, past, or current). The models also assessed the significance of interactions between biologic exposure ratio, biologic use at baseline, and time (i.e., calendar year) or disease duration. Statistical significance was defined as α = 0.05. All data management and analyses were conducted using SAS v9.4 (SAS, Cary, NC).
Results
Patient characteristics
The analysis included 1395 RA patients with a total of 6783 unique study visits from 2003 to 2015 (Table 2). Overall, 82.3% of patients were female. Mean (SD) age and disease duration at enrollment were 56.3 (14.2) years and 12.7 (11.9) years, respectively. Patients included in the analyses had an average of 4.9 study visits (range, 1–13 visits) during the study period. Most patients (66.4%) enrolled during the first 3 years after registry initiation, between 2003 and 2005. After the first year of the registry, returning patients comprised between 68.9% (in 2012) and 99.8% (in 2009) of the study sample.
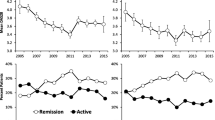
Over time, the proportion of patients with any medication use for RA in that year increased for biologics (from 41% in 2003 to 68% in 2015) and methotrexate (from 45 to 57%), and decreased for other nonbiologic DMARDs (from 36 to 25%), steroids (from 32 to 26%), and NSAIDs (from 61 to 41%) (Fig. 1). The biologic exposure ratio was calculated as the ratio of the patient’s total time on biologics, divided by their time in the BRASS registry. Using these calculations, mean (SD) biologic exposure ratio also increased during the study period, with a 1.6-fold increase from 0.41 (0.49) in 2003 to 0.65 (0.42) in 2015.
Disease activity: DAS28-CRP
Over time, DAS28-CRP scores consistently decreased as biologic exposure ratios increased (Fig. 2a). In the repeated measures regression model (Table 3), increased biologic exposure ratios were significantly associated with decreased DAS28-CRP score during follow-up (β = − 0.647; P < 0.001). Other statistically significant (P < 0.05) predictors of decreased DAS28-CRP score during follow-up were methotrexate use at baseline and study year, with increased effect size in later years of the study. Statistically significant (P < 0.05) predictors of increased DAS28-CRP score during follow-up were a higher baseline DAS28-CRP score, any biologic use at baseline or before enrollment, longer disease duration, a higher baseline mHAQ score, or currently smoking.
Disease activity, function impairment, and RA severity decreased over time with increased biologic exposure ratio. a Disease activity (Disease Activity Score-28 with C-reactive protein [DAS28-CRP]). b Function impairment (modified Health Assessment Questionnaire [mHAQ]). c RA severity (Routine Assessment of Patient Index Data [RAPID3]). Each data point represents the mean annual value ± standard error. See Table 2 for annual sample sizes
Functional status: mHAQ
Over time, mHAQ scores for function impairment decreased as biologic exposure ratios increased (Fig. 2b). In the repeated measures regression model (Table 3), increased biologic exposure ratios were significantly associated with decreased mHAQ score during follow-up (β = − 0.096; P < 0.001); methotrexate use at baseline also was a statistically significant (P < 0.05) predictor of decreased mHAQ score during follow-up. Statistically significant (P < 0.05) predictors of increased mHAQ score during follow-up were higher baseline mHAQ score, longer disease duration, or any biologic use at baseline.
RA severity: RAPID3
Over time, RAPID3 scores decreased as biologic exposure ratios increased (Fig. 2c). In the repeated measures regression model (Table 3), increased biologic exposure ratios were significantly associated with decreased RAPID3 score during follow-up (β = − 0.724; P < 0.001, where a biologic was not taken at baseline). Other statistically significant (P < 0.05) predictors of decreased RAPID3 score during follow-up were methotrexate use at baseline and completed graduate education. Statistically significant (P < 0.05) predictors of increased RAPID3 score during follow-up were a higher baseline mHAQ score, a higher baseline DAS28-CRP score, any biologic use before enrollment, currently smoking, and biologic exposure ratio × biologic use at baseline (β = 0.938; P = 0.024, for biologic exposure ratio where a biologic was taken at baseline).
Discussion
This study examined the effect of biologic exposure ratio, a measure of cumulative biologic use, on disease severity and patient functional status using longitudinal mixed-effects linear models in a sample of nearly 1400 RA patients in a prospective registry with up to 13 years of follow-up. From 2003 to 2015, as biologic use increased over time, there was a trend towards decreased disease activity (DAS28-CRP), decreased impairment of functional status (mHAQ), and decreased disease severity (RAPID3). A recent analysis of another RA disease registry reported similar results [36]. In that study, progressive decreases in Clinical Disease Activity Index (CDAI) and HAQ scores were observed with progressive increases in biologic use over time. Our study extends those results by showing that other measures of RA disease burden also improved during the period that biologic use increased. Our study also used repeated measures regression models to examine predictors for improved outcomes in RA patients. In these models, the biologic exposure ratio was a significant predictor of improved outcomes for RA disease activity, functional status, and disease severity. Previous research has shown that initiating biologic therapy in earlier RA (≤ 3 years) or in later RA (> 3 years) is associated with similar response rates and structural improvement, but patients with earlier RA report greater improvement in disability with biologic therapy compared with patients with later RA [7].
Several other covariates also were significant predictors of disease activity, function impairment, or disease severity. The convergence of disparate covariates across these models indicates that multiple factors in addition to biologic use may influence functional status in RA patients. For instance, methotrexate is a standard treatment for RA that is known to be effective in both biologic and nonbiologic treatment regimens [1]. In this study, any use of methotrexate in a given year was a significant predictor of improved patient outcomes across all models. Conversely, smoking is a known environmental risk factor [37,38,39], and “currently smoking” was a significant predictor of worsened disease activity and disease severity. Previous use of biologic agents, which is likely to identify patients with severe disease, as well as measures of function impairment and disease activity, were also found in patients with worse function impairment and disease activity over the course of the study.
Calendar year was a significant predictor of disease activity in the model for DAS28-CRP. Over the study period, the proportion of patients in a given year who used any biologic increased, with the greatest increase between 2003 and 2009. From 2010 to 2015, more than half of the patients received a biologic in any given year. Mean scores for DAS28-CRP decreased from 4.2 in the first year of the registry to below 2.6 (the cutoff for RA remission) only in the last 2 years of the analysis. In addition to possible dropouts of patients with higher disease activity, factors such as length of treatment and use of more biologics may have contributed to this finding. The relationship may be partially related to the increasing availability and use of biologic agents throughout the study period. As the BRASS registry does not include data on timing and duration of biologic use prior to enrollment, this analysis could not fully control for the duration of a patient’s lifetime biologic use or the potential effect of biologic use early versus late in disease progression. Although disease duration for the full sample was between 15 and 20 years throughout the course of the study, new enrollees in each annual population tended to have a slightly lower disease duration compared with returning patients. Further study into the long-term relationship between radiographic damage and biologic use is warranted, especially where the timing of biologic use in disease progression is known.
Comorbidities were not examined as possible predictors of disease activity in this analysis. Previous registry analyses have shown that the presence and number of comorbid conditions in RA are associated with lower chance of achieving remission [23, 40]. Assessment of outcomes of biologic therapy in real-world practice remains challenging due to the varied trajectory of disease across patients and the lack of a long-term control group, because patients whose disease progresses beyond nonbiologic DMARD management typically receive biologics. The BRASS database represents a rich source of data that includes 2700 variables composed of demographics, patient reported outcomes, and clinical data. This type of longitudinal data is uncommon and provides insight into the natural course of RA and its treatment over time. The registry sample, which primarily comprised middle-aged, Caucasian women with long-standing RA, was largely representative of the RA population and included both biologic-experienced and biologic-naïve patients.
As with all analyses, there were some notable limitations in this study, as follows. Mean disease duration at study entry was 12.7 years; thus, a substantial portion of active disease was not in the registry, especially during early disease when changes may be more rapid compared with established disease. The registry does not include data on disease severity at the time of biologic initiation or duration of biologic initiation prior to enrollment. Additional data on disease severity at the time of biologic initiation or duration of biologic initiation prior to enrollment in the registry may allow for additional relationships between biologic exposure and patient outcomes to be elucidated. The BRASS database only includes data from patients treated at the Robert Breck Brigham Arthritis Clinic. Previous research suggests that patients in the BRASS database who are less educated and have more severe disease activity are more likely to drop out of the registry [41]. Improvement of patient outcomes over time in this analysis may be explained, at least partially, by greater dropout for patients with more severe disease activity than those with less severe disease activity. The registry also lacks pharmacy data to confirm patient reports of drug utilization. Each of these may limit the external validity and generalizability of study findings to other populations. Multivariable analyses of clinical outcomes were conducted but there is a large potential for confounding from other factors not included in the study. Biologic adherence cannot be assessed from the BRASS registry database.
Biologic exposure may be affected by stopping and starting biologic therapy for many reasons, such as tolerability (i.e., infection), efficacy, insurance coverage, and patient preference. Discontinuation of biologic therapy for efficacy may include not only discontinuation for lack of efficacy but also for symptomatic response; patients who discontinue therapy when they feel better may need to restart biologic therapy when disease worsens again. Patient education may address these situations to improve patient adherence to prescribed biologic therapy.
In conclusion, greater cumulative exposure to biologics was significantly associated with improvements in disease activity, function impairment, and RA severity. Several other covariates also were significant predictors, indicating that various clinical and patient characteristics may contribute to the trajectory of RA. The findings from this study suggest that cumulative exposure to biologics may help to limit the effects of inflammation on patient outcomes over time. A causative effect of early versus late use of biologics was not directly assessed in this analysis. Future studies should examine whether earlier use of biologics is associated with improved long-term outcomes. Additional study of the potential effect of the timing of biologic treatment, including both duration of exposure and disease status at initiation, is needed to support the optimal use of biologics in the treatment of RA.
References
Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, Vaysbrot E, McNaughton C, Osani M, Shmerling RH, Curtis JR, Furst DE, Parks D, Kavanaugh A, O'Dell J, King C, Leong A, Matteson EL, Schousboe JT, Drevlow B, Ginsberg S, Grober J, St Clair EW, Tindall E, Miller AS, McAlindon T (2016) 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 68:1–26. https://doi.org/10.1002/art.39480
Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, Emery P, Gaujoux-Viala C, Gossec L, Nam J, Ramiro S, Winthrop K, de Wit M, Aletaha D, Betteridge N, Bijlsma JW, Boers M, Buttgereit F, Combe B, Cutolo M, Damjanov N, Hazes JM, Kouloumas M, Kvien TK, Mariette X, Pavelka K, van Riel PL, Rubbert-Roth A, Scholte-Voshaar M, Scott DL, Sokka-Isler T, Wong JB, van der Heijde D (2014) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 73:492–509. https://doi.org/10.1136/annrheumdis-2013-204573
Bykerk VP, Akhavan P, Hazlewood GS, Schieir O, Dooley A, Haraoui B, Khraishi M, Leclercq SA, Legare J, Mosher DP, Pencharz J, Pope JE, Thomson J, Thorne C, Zummer M, Bombardier C (2012) Canadian Rheumatology Association recommendations for pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs. J Rheumatol 39:1559–1582. https://doi.org/10.3899/jrheum.110207
Singh JA, Hossain A, Tanjong Ghogomu E, Kotb A, Christensen R, Mudano AS, Maxwell LJ, Shah NP, Tugwell P, Wells GA (2016) Biologics or tofacitinib for rheumatoid arthritis in incomplete responders to methotrexate or other traditional disease-modifying anti-rheumatic drugs: a systematic review and network meta-analysis. Cochrane Database Syst Rev CD012183. doi:https://doi.org/10.1002/14651858.CD012183
Hallert E, Husberg M, Bernfort L (2012) The incidence of permanent work disability in patients with rheumatoid arthritis in Sweden 1990-2010: before and after introduction of biologic agents. Rheumatology (Oxford) 51:338–346. https://doi.org/10.1093/rheumatology/ker332
Krishnan E, Lingala B, Bruce B, Fries JF (2012) Disability in rheumatoid arthritis in the era of biological treatments. Ann Rheum Dis 71:213–218. https://doi.org/10.1136/annrheumdis-2011-200354
Baumgartner SW, Fleischmann RM, Moreland LW, Schiff MH, Markenson J, Whitmore JB (2004) Etanercept (Enbrel) in patients with rheumatoid arthritis with recent onset versus established disease: improvement in disability. J Rheumatol 31:1532–1537
Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, van Zeben D, Kerstens PJ, Hazes JM, Zwinderman AH, Ronday HK, Han KH, Westedt ML, Gerards AH, van Groenendael JH, Lems WF, van Krugten MV, Breedveld FC, Dijkmans BA (2005) Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 52:3381–3390. https://doi.org/10.1002/art.21405
Quinn MA, Conaghan PG, O'Connor PJ, Karim Z, Greenstein A, Brown A, Brown C, Fraser A, Jarret S, Emery P (2005) Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomized, double-blind, placebo-controlled trial. Arthritis Rheum 52:27–35. https://doi.org/10.1002/art.20712
Neovius M, Simard JF, Klareskog L, Askling J (2011) Sick leave and disability pension before and after initiation of antirheumatic therapies in clinical practice. Ann Rheum Dis 70:1407–1414. https://doi.org/10.1136/ard.2010.144139
Weinblatt ME, Bathon JM, Kremer JM, Fleischmann RM, Schiff MH, Martin RW, Baumgartner SW, Park GS, Mancini EL, Genovese MC (2011) Safety and efficacy of etanercept beyond 10 years of therapy in North American patients with early and longstanding rheumatoid arthritis. Arthritis Care Res (Hoboken) 63:373–382. https://doi.org/10.1002/acr.20372
Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, Paulus HE, Mudano A, Pisu M, Elkins-Melton M, Outman R, Allison JJ, Suarez Almazor M, Bridges SL Jr, Chatham WW, Hochberg M, MacLean C, Mikuls T, Moreland LW, O'Dell J, Turkiewicz AM, Furst DE (2008) American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 59:762–784. https://doi.org/10.1002/art.23721
Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, Moreland LW, O'Dell J, Winthrop KL, Beukelman T, Bridges SL Jr, Chatham WW, Paulus HE, Suarez-Almazor M, Bombardier C, Dougados M, Khanna D, King CM, Leong AL, Matteson EL, Schousboe JT, Moynihan E, Kolba KS, Jain A, Volkmann ER, Agrawal H, Bae S, Mudano AS, Patkar NM, Saag KG (2012) 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 64:625–639. https://doi.org/10.1002/acr.21641
Harrold LR, Litman HJ, Connolly SE, Kelly S, Hua W, Alemao E, Rosenblatt L, Rebello S, Kremer JM (2017) A window of opportunity for abatacept in RA: is disease duration an independent predictor of low disease activity/remission in clinical practice? Clin Rheumatol 36:1215–1220. https://doi.org/10.1007/s10067-017-3588-7
Barra L, Pope JE, Payne M (2009) Real-world anti-tumor necrosis factor treatment in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: cost-effectiveness based on number needed to treat to improve health assessment questionnaire. J Rheumatol 36:1421–1428. https://doi.org/10.3899/jrheum.081122
Dewitt EM, Li Y, Curtis JR, Glick HA, Greenberg JD, Anstrom KJ, Kremer JM, Reed G, Schulman KA, Reed SD (2013) Comparative effectiveness of nonbiologic versus biologic disease-modifying antirheumatic drugs for rheumatoid arthritis. J Rheumatol 40:127–136. https://doi.org/10.3899/jrheum.120400
Chen JS, Makovey J, Lassere M, Buchbinder R, March LM (2014) Comparative effectiveness of anti-tumor necrosis factor drugs on health-related quality of life among patients with inflammatory arthritis. Arthritis Care Res (Hoboken) 66:464–472. https://doi.org/10.1002/acr.22151
Thorne C, Bensen WG, Choquette D, Chow A, Khraishi M, Atkins CJ, Kelsall JT, Lehman AJ, Shawi M, Khalil H, Nantel F, Rampakakis E, Sampalis JS, Otawa S (2014) Effectiveness and safety of infliximab in rheumatoid arthritis: analysis from a Canadian multicenter prospective observational registry. Arthritis Care Res (Hoboken) 66:1142–1151. https://doi.org/10.1002/acr.22290
Richter A, Strangfeld A, Herzer P, Wilden E, Bussmann A, Listing J, Zink A (2014) Sustainability of rituximab therapy in different treatment strategies: results of a 3-year followup of a German biologics register. Arthritis Care Res (Hoboken) 66:1627–1633. https://doi.org/10.1002/acr.22327
De Keyser F, Hoffman I, Durez P, Kaiser MJ, Westhovens R (2014) Longterm followup of rituximab therapy in patients with rheumatoid arthritis: results from the Belgian MabThera in Rheumatoid Arthritis registry. J Rheumatol 41:1761–1765. https://doi.org/10.3899/jrheum.131279
Courvoisier DS, Alpizar-Rodriguez D, Gottenberg JE, Hernandez MV, Iannone F, Lie E, Santos MJ, Pavelka K, Turesson C, Mariette X, Choquette D, Hetland ML, Finckh A (2016) Rheumatoid arthritis patients after initiation of a new biologic agent: trajectories of disease activity in a large multinational cohort study. EBioMedicine 11:302–306. https://doi.org/10.1016/j.ebiom.2016.08.024
Pappas DA, Kremer JM, Griffith J, Reed G, Salim B, Karki C, Garg V (2017) Long-term effectiveness of adalimumab in patients with rheumatoid arthritis: an observational analysis from the Corrona rheumatoid arthritis registry. Rheumatol Ther 4:375–389. https://doi.org/10.1007/s40744-017-0077-z
Armagan B, Sari A, Erden A, Kilic L, Erdat EC, Kilickap S, Kiraz S, Bilgen SA, Karadag O, Akdogan A, Ertenli I, Kalyoncu U (2018) Starting of biological disease modifying antirheumatic drugs may be postponed in rheumatoid arthritis patients with multimorbidity: single center real life results. Medicine (Baltimore) 97:e9930. https://doi.org/10.1097/MD.0000000000009930
Asai S, Fujibayashi T, Oguchi T, Hanabayashi M, Hayashi M, Matsubara H, Ito T, Yabe Y, Watanabe T, Hirano Y, Kanayama Y, Kaneko A, Kato T, Takagi H, Takahashi N, Funahashi K, Takemoto T, Asai N, Watanabe T, Ishiguro N, Kojima T (2018) Predictors of biologic discontinuation due to insufficient response in patients with rheumatoid arthritis who achieved clinical remission with biologic treatment: a multicenter observational cohort study. Mod Rheumatol 28:221–226. https://doi.org/10.1080/14397595.2017.1332558
Brown LE, Frits ML, Iannaccone CK, Weinblatt ME, Shadick NA, Liao KP (2015) Clinical characteristics of RA patients with secondary SS and association with joint damage. Rheumatology (Oxford) 54:816–820. https://doi.org/10.1093/rheumatology/keu400
Curtis JR, van der Helm-van Mil AH, Knevel R, Huizinga TW, Haney DJ, Shen Y, Ramanujan S, Cavet G, Centola M, Hesterberg LK, Chernoff D, Ford K, Shadick NA, Hamburger M, Fleischmann R, Keystone E, Weinblatt ME (2012) Validation of a novel multibiomarker test to assess rheumatoid arthritis disease activity. Arthritis Care Res (Hoboken) 64:1794–1803. https://doi.org/10.1002/acr.21767
Iannaccone CK, Lee YC, Cui J, Frits ML, Glass RJ, Plenge RM, Solomon DH, Weinblatt ME, Shadick NA (2011) Using genetic and clinical data to understand response to disease-modifying anti-rheumatic drug therapy: data from the Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study. Rheumatology (Oxford) 50:40–46. https://doi.org/10.1093/rheumatology/keq263
Lillegraven S, Prince FH, Shadick NA, Bykerk VP, Lu B, Frits ML, Iannaccone CK, Kvien TK, Haavardsholm EA, Weinblatt ME, Solomon DH (2012) Remission and radiographic outcome in rheumatoid arthritis: application of the 2011 ACR/EULAR remission criteria in an observational cohort. Ann Rheum Dis 71:681–686. https://doi.org/10.1136/ard.2011.154625
Plenge RM, Cotsapas C, Davies L, Price AL, de Bakker PI, Maller J, Pe'er I, Burtt NP, Blumenstiel B, DeFelice M, Parkin M, Barry R, Winslow W, Healy C, Graham RR, Neale BM, Izmailova E, Roubenoff R, Parker AN, Glass R, Karlson EW, Maher N, Hafler DA, Lee DM, Seldin MF, Remmers EF, Lee AT, Padyukov L, Alfredsson L, Coblyn J, Weinblatt ME, Gabriel SB, Purcell S, Klareskog L, Gregersen PK, Shadick NA, Daly MJ, Altshuler D (2007) Two independent alleles at 6q23 associated with risk of rheumatoid arthritis. Nat Genet 39:1477–1482. https://doi.org/10.1038/ng.2007.27
Shadick NA, Cook NR, Karlson EW, Ridker PM, Maher NE, Manson JE, Buring JE, Lee IM (2006) C-reactive protein in the prediction of rheumatoid arthritis in women. Arch Intern Med 166:2490–2494. https://doi.org/10.1001/archinte.166.22.2490
Solomon DH, Finkelstein JS, Shadick N, LeBoff MS, Winalski CS, Stedman M, Glass R, Brookhart MA, Weinblatt ME, Gravallese EM (2009) The relationship between focal erosions and generalized osteoporosis in postmenopausal women with rheumatoid arthritis. Arthritis Rheum 60:1624–1631. https://doi.org/10.1002/art.24551
Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL (1995) Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 38:44–48. https://doi.org/10.1002/art.1780380107
van Gestel AM, Haagsma CJ, van Riel PL (1998) Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum 41:1845–1850. https://onlinelibrary.wiley.com/doi/abs/10.1002/1529-0131(199810)41:10%3C1845::AID-ART17%3E3.0.CO%3B2-K
Pincus T, Summey JA, Soraci SA Jr, Wallston KA, Hummon NP (1983) Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum 26:1346–1353. https://doi.org/10.1002/art.1780261107
Pincus T, Yazici Y, Bergman MJ (2009) RAPID3, an index to assess and monitor patients with rheumatoid arthritis, without formal joint counts: similar results to DAS28 and CDAI in clinical trials and clinical care. Rheum Dis Clin N Am 35:773–778, viii. https://doi.org/10.1016/j.rdc.2009.10.008
Kavanaugh A, Singh R, Karki C, Etzel CJ, Kremer JM, Greenberg JD, Griffith J (2018) Disease activity and biologic use in patients with psoriatic arthritis or rheumatoid arthritis. Clin Rheumatol 37:2275–2280. https://doi.org/10.1007/s10067-018-4140-0
Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, Ronnelid J, Harris HE, Ulfgren AK, Rantapaa-Dahlqvist S, Eklund A, Padyukov L, Alfredsson L (2006) A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum 54:38–46. https://doi.org/10.1002/art.21575
Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L (2004) A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum 50:3085–3092. https://doi.org/10.1002/art.20553
Stolt P, Bengtsson C, Nordmark B, Lindblad S, Lundberg I, Klareskog L, Alfredsson L, group Es (2003) Quantification of the influence of cigarette smoking on rheumatoid arthritis: results from a population based case-control study, using incident cases. Ann Rheum Dis 62:835–841. https://doi.org/10.1136/ard.62.9.835
Ranganath VK, Maranian P, Elashoff DA, Woodworth T, Khanna D, Hahn T, Sarkisian C, Kremer JM, Furst DE, Paulus HE (2013) Comorbidities are associated with poorer outcomes in community patients with rheumatoid arthritis. Rheumatology (Oxford) 52:1809–1817. https://doi.org/10.1093/rheumatology/ket224
Iannaccone CK, Fossel A, Tsao H, Cui J, Weinblatt M, Shadick N (2013) Factors associated with attrition in a longitudinal rheumatoid arthritis registry. Arthritis Care Res (Hoboken) 65:1183–1189. https://doi.org/10.1002/acr.21940
Acknowledgments
Jonathan Latham (PharmaScribe, LLC, on behalf of Amgen Inc.), Dikran Toroser (Amgen Inc.), and Hafiz Oko-osi (Amgen Inc.) provided medical writing assistance.
Funding
This analysis was funded by Amgen Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
At the time of the analysis, B.S.S., D.C., and A.M. were employees and stockholders of Amgen Inc. N.M.G. and B.L.B. were employees of Health Analytics, LLC, which received funding from Amgen Inc. to conduct the study. N.A.S. has been a consultant to Bristol-Myers Squibb and has received research grants from Amgen, Mallinckrodt, UCB, Crescendo Biosciences, Sanofi, Bristol-Myers Squibb, and DxTerity. M.E.W. has been a consultant to Amgen, Bristol-Myers Squibb, Crescendo Bioscience, and UCB and has received research grants from Amgen, Bristol-Myers Squibb, Crescendo Bioscience, UCB, DxTerity, and Sanofi/Regeneron. M.L.F., C.I, and J.C declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Shadick, N.A., Gerlanc, N.M., Frits, M.L. et al. The longitudinal effect of biologic use on patient outcomes (disease activity, function, and disease severity) within a rheumatoid arthritis registry. Clin Rheumatol 38, 3081–3092 (2019). https://doi.org/10.1007/s10067-019-04649-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04649-4