Abstract
Background
Medication adherence is suboptimal in rheumatoid arthritis (RA) patients and impacts outcomes. DMARD-free remission (DFR) is a sustainable and achievable outcome in a minority of RA patients. Different factors have been associated with DFR, although persistence in therapy (PT), a component of the adherence construct, has never been examined. The study’s primary aim was to investigate the impact of PT’s characteristics on DFR in a cohort of Hispanic patients with recent-onset RA.
Methods
A single data abstractor reviewed the charts from 209 early (symptoms duration ≤ 1 year) RA patients. All the patients had prospective assessments of disease activity and PT and at least 1 year of follow-up, which was required for the DFR definition. DFR was defined when patients achieved ≥ 1 year of continuous Disease Activity Score-28 joints evaluated ≤ 2.6, without DMARDs and corticosteroids. PT was defined based on pre-specified criteria and recorded through an interview from 2004 to 2008 and thereafter through a questionnaire. Cases (patients who achieved ≥ 1 DFR status) were paired with controls (patients who never achieved DFR during their entire follow-up) according to ten relevant variables (1:2). Cox regression analysis estimated hazard ratios (HRs) for DFR according to two characteristics of PT: the % of the patient follow-up PT and early PT (first 2 years of patients’ follow-up).
Results
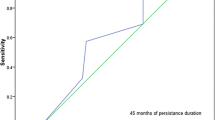
In March 2022, the population had 112 (55–181) patient/years follow-up. There were 23 patients (11%) with DFR after 74 months (44–122) of follow-up, and the DFR status was maintained during 48 months (18–82). Early PT was associated with DFR, while the % of the patient follow-up PT was not: HR = 3.84 [1.13–13.07] when the model was adjusted for cumulative N of DMARDs/patient and 3.16 [1.14–8.77] when also adjusted for baseline SF-36 physical component score. A lower N of cumulative DMARDs/patient was also retained in the models. Receiving operating curve to define the best cutoff of patient follow-up being PT to predict DFR was 21 months: sensitivity of 0.739, specificity of 0.717, and area under the curve of 0.682 (0.544–0.821).
Conclusions
DFR status might be added to the benefits of adhering to prescribed treatment.
Similar content being viewed by others
Background
Current treatment guidelines for rheumatoid arthritis (RA) suggest tapering disease-modifying anti-rheumatic drugs (DMARDs) when patients are in sustained remission (SR) [1]. Two recent systematic literature and narrative reviews inform that with a treat-to-target approach, an increasing number of patients might achieve and sustain DMARD-free remission (DFR), with frequencies ranging from 3.6 to 22% of the patients [2, 3]. (Sustained) DFR has nowadays considered the closest state to RA cure [4] and, from the patient perspective, has been associated with the reversal of functional disability and the resolution of symptoms such as fatigue [5]. Several factors had been demonstrated to predict the successful maintenance of remission after classical DMARDs withdrawal. The absence of disease-specific autoantibodies is the most consistent and best predictor [2, 3, 6]. Meanwhile, symptom duration has arisen conflicting results, which might be explained by a non-linear association with DFR but refined to a short period, the window of opportunity [2, 3, 7]. Additional factors identified are a longer duration of SR before the drug withdrawal [8], lower disease activity at the time of treatment cessation [9,10,11,12], using methotrexate as the last DMARD before withdrawal [9], circulating inflammatory biomarkers and peripheral CD4+ T-cell gene expression [11], and a model that combined RA quality of life (QoL) score, musculoskeletal ultrasound-derived information, and the percentage of inflammation-related T-cell [13].
Tapering DMARDs, notably methotrexate, is desirable for RA patients concerned about long-term side effects and the burden of taking tablets or self-injecting if they are well [14]. These impact patient adherence to the prescribed treatment, which reflects the extent to which patients take their medication as prescribed [15, 16]. Previous literature reviews highlight that among patients with RA, adherence to DMARDs is suboptimal, and poor adherence affects 20 to 70% of the patients during their follow-up [17,18,19,20,21]. Inadequate medication adherence includes three major components (persistence, initiation adherence, and execution adherence) and causes a negative impact on the different patient and physician-reported outcomes, which our group has confirmed in Hispanic RA patients [17,18,19,20,21,22,23,24,25]. Meanwhile, the impact of medication adherence and its components on DFR, a realistic and achievable goal, has not been previously examined.
The study aimed to investigate the impact of persistence on therapy (PT)’s characteristics on DFR in an inception and ongoing cohort of Hispanic patients with recent-onset RA. We additionally described the DFR phenomenon.
Methods
Setting and study population
Patients with RA were identified from the early arthritis clinic of a national referral center for rheumatic diseases located in Mexico City, the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán. Patients entering the clinic had been previously described [23,24,25]. They had a disease duration of less than a year when first evaluated and were assessed every 2 months during the first 2 years of follow-up. After that, visits were scheduled every 2, 4, or 6 months, depending on the patients and disease characteristics.
Treatment was prescribed by the rheumatologist in charge of the clinic, who embraced paradigmatic changes in treating the disease over the years [26, 27]. During the first years, she/he adopted aggressive and tight control of inflammation, considering the critical nature of the early disease and the functional deterioration due to treatment delay [26, 28]. A decade later, this concept was refined and formulated as the “treat-to-target” (T2T)-oriented approach [27] and was incorporated into patient management.
At study entry, the primary rheumatologist recorded a complete medical history, demographic data, and disease-specific auto-antibodies (rheumatoid factor [RF] and antibodies to citrullinated proteins [ACPA]). Standardized follow-up evaluations included extended joint counts, patient (PROs)- and physician-reported outcomes, comorbidity, treatment assessment, laboratory parameters, and hand and feet X-rays (on an annual basis) [23,24,25].
From the beginning of the clinic (2004), the primary rheumatologist prospectively assessed the patient’s medication behavior in standardized formats. From 2004 to 2008, she/he evaluated persistence through an interview conducted at every visit. Patients reported the names, doses, and schedules of DMARDs and corticosteroids they had taken since the previous visit. Then, patients were asked about missing and incorrect medications, quantities, and schedules. The rheumatologist compared the last prescription and the actual treatment and recorded the number of days of missing drugs. From 2008 onwards, persistence in therapy was assessed through the compliance questionnaire, with good sensitivity and satisfactory specificity to detect persistence [23].
Study design
A nested within a cohort case-control study was designed to accomplish the primary objective. Cases were defined as patients who achieved at least one DFR status and controls as patients who never achieved DFR during their entire follow-up. Controls were paired to cases (2:1) according to sex, age at RA diagnosis (± 15 years), education, socioeconomic status, presence of RF and APCA at baseline, baseline Disease Activity Score-28 joints evaluated (DAS28) European League Against Rheumatism (EULAR) category [29], corticosteroid use during the first year of follow-up, number of DMARDs/patient during the first year, and baseline erosions.
Data collection
Initiating in January 2022 and ending in March 2022, a single and trained data abstractor retrospectively reviewed all the charts and corroborated the integrity of the data collected. An independent observer confirmed all the DFR periods and PT statuses.
Definitions
DFR status was defined when patients achieved SR (at least 1 year of continuous follow-up clinical assessments with DAS28 ≤ 2.6) without concomitant DMARDs and corticosteroids (any route). The definition combined characteristics from previously published definitions [2, 3].
According to the interview, non-PT was defined as the omission for ≥ 7 consecutive days of at least one DMARD and corticosteroids. Regarding methotrexate, at least one missing weekly dose was considered non-persistence. According to the compliance questionnaire [23], a patient was considered to be non-PT if in item 10 (“In the past 6 months, how often do you completely stop taking your DMARDs?”), boxes 2 (“sometimes”), 3 (“almost always”), and 4 (“always”) were filled.
Statistical analysis
Descriptive statistics were used. The χ2 and Mann-Whitney U tests were used to compare the non-normally distributed variables according to their category.
For each patient, PT was calculated as the percentage of the entire patient follow-up (up to the first DFR status for cases or equivalent for controls) that he/she was non-PT-free. Also, early PT was defined if continuous PT during the first 2 years of follow-up was identified.
Cox proportional hazard regression analysis was used to estimate the hazard ratios (HRs) and their respective 95% confidence interval (CI) for DFR according to PT’s characteristics. Two PT characteristics were included: % of the patient follow-up PT and/or early PT. We initially performed a univariate analysis and then performed a multivariate analysis to adjust for potential confounders (a p cutoff ≤ 0.05 in the univariate analysis was considered to include the variables): model 1 was adjusted for baseline 36-Item Short Form Survey (SF-36) physical component score, model 2 was adjusted for the cumulative number of DMARDs/patient during the first 2 years of follow-up, and model 3 included early PT and was adjusted for baseline SF-36 physical component score and number of DMARDs/patient. We also switched in the models the % of the patient’s follow-up PT to the patient achievement of PT ≥ 80% of his/her follow-up.
Receiving operating curve (ROC) was used to define the best cutoff for continuous patient follow-up on PT to achieve (the first) DFR status.
All analyses were performed using SPSS (version 21.0, IBM Corp., Armonk, NY, USA).
Ethics
The study was approved by the Intutional Review Board (Comités de Ética e Investigación del Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán). All the patients provided written informed consent for clinical follow-ups when entering the clinic. They provided additional written permission to review each patient’s chart and present data in scientific publications.
Results
Study population characteristics
Up to March 2022, 237 patients had been evaluated in the early arthritis clinic, and 223 had at least 1 year of follow-up, which was required as per the DFR definition. Fourteen patients had a different diagnosis (to recent-onset RA) and were eliminated from the analysis (Fig. 1).
The baseline patient characteristics of the remaining 209 patients are summarized in Table 1. Briefly, they were primarily middle-aged women with medium-low socioeconomic status. The majority of them had RF and ACPA, while a few had baseline erosions. Patients had significant clinical and serological disease activity, translating into disability and poor quality of life (QoL). In addition, all the patients have indicated DMARDs, and almost half of them have corticosteroids.
Description of DFR phenomenon
Up to March 2022, the early arthritis clinic had 112 (55–181) patient/years follow-up. There were 23 patients (11%) who achieved DFR status (cases) after 74 months (44–122) of follow-up; in them, DFR status was maintained during 48 months (18–82). Their DAS28 at DFR was 1.7 (1–2.1), and 20 of them (87%) additionally had Boolean remission and according to the Simplified Disease Activity Index (SDAI) [30].
Figure 2 summarizes the DFR behavior of the 23 cases. Six cases (26.1%) presented health care drop-out while in DFR, and six additional patients (26.1%) are currently active in the early arthritis clinic and maintain their DFR status. The remaining 11 patients (47.8%) lost DFR status; four patients had health care drop-outs, while seven are currently active in the early arthritis clinic. We further compared baseline characteristics between patients who lost DFR status and their counterparts, and no significant differences were identified (Additional file 1: Table S1).
Comparison of cases and controls
The 23 cases were paired with 46 controls. Additional file 2: Table S2 summarizes the matching criteria, and the percentage of case-controls matching achieved.
Cases and controls were similar in baseline characteristics, but the SF36 physical component score was lower among the cases (Table 1).
Table 2 summarizes the comparison of cumulative (up to DFR status for cases or equivalent for controls) DAS28 joints evaluated [31], the number of DMARDs/patient, and PT characteristics between cases and controls. As observed, cases received a lower number of DMARDs/patient and were more frequently early PT when compared to controls; also, cases tend to be more frequently PT than controls.
Impact of PT on DFR phenomenon
Table 3 summarizes the results from Cox proportional hazard regression analysis. In models 2 and 3, early PT (HR 3.84 [1.13–13.07] and 3.16 [1.14–8.77], respectively) and a lower number of DMARDs/patient during the first 2 years of follow-up (HR 0.19 [0.08–0.45] and 0.12 [0.07–0.41], respectively) were associated with DFR status.
Finally, ROC to define the best cutoff of patient follow-up being PT to predict DFR was 21 months: sensitivity of 0.739, specificity of 0.717, and AUC of 0.682 (0.544–0.821), as shown in Fig. 3.
Discussion
Hispanic RA patients present distinctive characteristics such as a lower age at presentation, an extreme female preponderance, and a less severe disease expression; however, they are frequently uninsured with low socioeconomic status and lesser educated than patients from developed countries [32, 33]. These are known variables to impact patients’ commitment to the prescribed treatment. Published observations have shown that poor adherence to therapy is a generalized phenomenon among RA patients, potentially reversible, and associated with a wide variety of unfavorable outcomes [15]. Most relevant include increased disease activity, disease flares, and health care drop-outs; worse PROs (such as increased pain, disability, and reduced QoL); and decreased rates of remission [16,17,18,19,20,21,22,23,24,25, 34,35,36,37,38,39,40]. In contrast, PT translates into long-standing significant improvements, which additionally appear early on [24].
The present study involved a well-characterized inception ongoing cohort of Hispanic patients with early RA. Patients had a long-term standardized follow-up, which included the prospective assessment of PT (a component of the adherence construct), disease activity-related outcomes, and current treatment. Traditional DMARDs given according to a T2T strategy were the mainstay of treatment. In addition, we followed cohort methodological recommendations to ensure the quality of the data [41]. Accordingly, we consider the results to reflect patients’ daily conditions and are of practical relevance.
The study complements the current knowledge on the topic and extends the benefits of the patients’ adherence to DMARDs to DFR status. In particular, early PT, which was defined as occurring during the first 2 years of patients’ cohort enrollment, was associated with DFR, while the percentage of the patient’s follow-up PT was not. Interestingly, 21 months was the best cutoff of the patient’s follow-up PT to predict DFR. The results are in accordance with the window of opportunity concept that states there are superior clinical responses and the potential for remission (we might extend to DFR) when patients with RA are managed early and aggressively with DMARDs [42,43,44,45]. Also, the 21-month cutoff highlights that patients need to adhere (closely and) early on during their follow-up to treatment recommendations if significant outcomes are desired. In regard to the predictors of DFR, different factors have been described, although PT has never been examined [2, 3, 6, 12, 45, 46]. Genetical [2], immunological [2, 3], imaging [3], and clinical associations [3] with predictors of DFR have been consistently identified, including the presence of autoantibodies, absence of power Doppler signal on musculoskeletal ultrasound, lower disease activity, and PROs at treatment cessation. Our study design is unique in addressing such a hypothesis, while we could not confirm additional factors. Moreover, some authors argued that the constant frequency of DFR in different cohorts of patients over time suggests it most likely represents spontaneous remission without any direct relationship to treatment [6]. However, adherence constructs had been related to different outcomes, including SR, which highlights results plausibility [43].
A lower N of DMARDs/patient during the first 2 years of follow-up was retained in the model. It could be explained by patients with DFR perceived by the treating rheumatologist with lower disease activity at baseline and might be indicated a lesser intensive treatment. There are circumstances where clinicians disagree with the disease activity assessment from a composite index and, therefore, would prefer to make a treatment decision based on their clinical judgment [47]. Also, patients were treated with a T2T approach, a complex process involving aggressive early management with several therapy modifications requiring frequent close monitoring of disease activity and drug toxicities, and more liable to suboptimal adherence in real-life clinical practice [47]. More complex therapeutic regimens have been associated with lower compliance, confirmed in our population [24].
Finally, we observed that DFR was achieved by 11% of the patients after 74 months of follow-up; also, DFR was sustained for 48 months, while almost half of the patients flared subsequently. Our prevalence figure for DFR status follows literature reviews and overviews that include data from early arthritis cohorts [2, 3, 6, 12, 45, 46]. Also, substantial DFR durations [9, 48] and a similar percentage of flares [45, 49, 50] had been previously described in patients who achieved DFR status. Finally, we did not identify baseline differences between patients who maintained DFR and their counterparts, which was probably related to the limited number of patients in either group.
Some limitations need to be addressed. First, the study was performed in a RA population with distinct characteristics, limiting the results’ generalization. Second, the power of the study was limited by the occurrence of only 23 cases which allows 2–3 potential predictors per model. Third, we did not use a single validated questionnaire to assess PT and arbitrarily used a lag time of 1 week to define therapy discontinuation; nonetheless, our rate of non-PT was consistent with similar measures in related studies [25, 35, 51, 52]. Fourth, we did not consider physician adherence to the T2T strategy, associated with better outcomes, such as higher remission rates and improved disease activity. Lastly, DFR status was defined based on sustained DAS28 cutoff, which might not reflect a real (sustained) remission status.
Conclusions
In summary, DFR is the most desirable outcome for RA patients and a proxy for disease cure. It can be achieved in a real-world setting, in a significant number of Hispanic patients with the recent-onset disease, and treated with a T2T approach. The current study extends the benefits of adhering to the prescribed treatment to DFR status and highlights that persistence with prescribed therapy during the first 21 months of disease follow-up might be associated with the most relevant outcome.
Availability of data and materials
All data that support our findings are contained within the manuscript. Requests for further details on the dataset and queries related to data sharing arrangements may be submitted to the corresponding author.
Abbreviations
- RA:
-
Rheumatoid arthritis
- DMARDs:
-
Disease-modifying anti-rheumatic drugs
- SR:
-
Sustained remission
- DFR:
-
DMARD-free remission
- QoL:
-
Quality of life
- PT:
-
Persistence in therapy
- T2T:
-
Treat-to-target
- RF:
-
Rheumatoid factor
- ACPA:
-
Antibodies to citrullinated proteins
- PROs:
-
Patient-reported outcomes
- DAS28:
-
Disease Activity Score-28 joints evaluated
- EULAR:
-
European League Against Rheumatism
- HRs:
-
Hazard ratios
- CI:
-
Confidence interval
- SF-36:
-
36-Item Short Form Survey
- ROC:
-
Receiving operating curve
- SDAI:
-
Simplified Disease Activity Index
References
Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. J Ann Rheum Dis. 2020;79:685–99.
Verstappen M, van Mulligen E, de Jong PHP, van der Helm-van Mil AHM. DMARD-free remission as novel treatment target in rheumatoid arthritis: a systematic literature review of achievability and sustainability. RMD Open. 2020;6:1–13.
Gul H, Harnden K, Saleem BL. Defining the optimal strategies for achieving drug-free remission in rheumatoid arthritis: a narrative review. Healthcare. 2021;9:1–20.
Filipowicz-Sosnowska A. Drug-free remission: the goal of the future in management of patients with rheumatoid arthritis. Reumatologia. 2017;55:284–9.
Ajeganova S, van Steenbergen HW, van Nies JA, Hamburguesas LE, Huizinga TWJ, van der Helm-van Mil AHM. Disease-modifying antirheumatic drug-free sustained remission in rheumatoid arthritis: an increasingly achievable outcome with subsidence of disease symptoms. Ann Rheum Dis. 2016;75:867–73.
Scott IC, Kingsley GH, Scott DL. Can we discontinue synthetic disease-modifying anti-rheumatic drugs in rheumatoid arthritis? Clin Exp Rheumatol. 2013;31(Suppl):S4–8.
van Nies JA, Tsonaka R, Gaujoux-Viala C, Fautrel B, van der Helm-van Mil AHM. Evaluating relationships between symptom duration and persistence of rheumatoid arthritis: does a window of opportunity exist? Results on the Leiden early arthritis clinic and ESPOIR cohorts. Ann Rheum Dis. 2015;74:806–12.
Brown AK, Conaghan PG, Karim Z, Quinn MA, Ikeda K, Peterfy CG, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58:2958–67.
Klarenbeek NB, van der Kooij SM, Guler-Yuksel M, van Groenendael JH, Han KH, Kerstens PJ, et al. Discontinuing treatment in patients with rheumatoid arthritis in sustained clinical remission: exploratory analyses from the BeSt study. Ann Rheum Dis. 2011;70:315–9.
Kuijper TM, Luime JJ, de Jong PH, Gerards AH, van Zeben D, Tchetverikov I, et al. Tapering conventional synthetic DMARDs in patients with early arthritis in sustained remission: 2-year follow-up of the tREACH trial. Ann Rheum Dis. 2016;75:2119–23.
Baker KF, Skelton AJ, Lendrem DW, Scadeng A, Thompson B, Pratt AG, et al. Predicting drug-free remission in rheumatoid arthritis: a prospective interventional cohort study. J Autoimmun. 2019;105:1–8.
van der Woude D, Young A, Jayakumar K, Mertens BJ, Toes RE, van der Helm-van Mil AH, et al. Prevalence of and predictive factors for sustained disease-modifying antirheumatic drug-free remission in rheumatoid arthritis: results from two large early arthritis cohorts. Arthritis Rheum. 2009;60:2262–71.
Gul H, Ponchel F, Emery P. In RA patients in remission, which biomarkers predict successful tapering of csDMARDs? Ann Rheum Dis. 2021;80(Suppl 1):110.
Hughes LD, Done J, Young A. A 5-item version of the Compliance Questionnaire for Rheumatology (CQR5) successfully identifies low adherence to DMARDs. BMC Musculoskelet Disord. 2013;14:286.
Murage MJ, Tongbram V, Feldman SR, Malatestinic WN, Larmore CJ, Muram TM, et al. Medication adherence and persistence in patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis: a systematic literature review. Patient Prefer Adherence. 2018;12:1483–503.
van den Bemt BJ, Zwikker HE, van den Ende CH. Medication adherence in patients with rheumatoid arthritis: a critical appraisal of the existing literature. Expert Rev Clin Immunol. 2012;8:337–51.
Pasma A, van’t Spijker A, Hazes JMW, Busschbach JJV, Luime JJ. Factors associated with adherence to pharmaceutical treatment for rheumatoid arthritis patients: a systematic review. Semin Arthritis Rheum. 2013;43:18–28.
Salt E, Frazier SK. Adherence to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: a narrative review of the literature. Orthop Nurs. 2010;29:260–75.
Blum MA, Koo D, Doshi JA. Measurement and rates of persistence with and adherence to biologics for rheumatoid arthritis: a systematic review. Clin Ther. 2011;33:901–13.
Gossec L, Tubach F, Dougados M, Ravaud P. Reporting of adherence to medication in recent randomized controlled trials of 6 chronic diseases: a systematic literature review. Am J Med Sci. 2007;334:248–54.
De Achaval S, Suarez-Almazor ME. Treatment adherence to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and systemic lupus erythematosus. Int J Clin Rheumatol. 2010;5:313–26.
Viller F, Guillemin F, Briançon S, Moum T, Suurmeijer T, van den Heuvel W. Compliance with drug therapy in rheumatoid arthritis. A longitudinal European study. Joint Bone Spine. 2000;67:178–82.
Contreras-Yañez I, Ponce-De Leon S, Cabiedes J, Rull-Gabayet M, Pascual-Ramos V. Inadequate therapy behavior is associated to disease flares in patients with rheumatoid arthritis who have achieved remission with disease-modifying anti-rheumatic drugs. Am Med Sci. 2010;340:282–90.
Contreras-Yáñez I, Cabiedes J, Villa AR, Rull-Gabayet M, Pascual-Ramos V. Persistence on therapy is a major determinant of patient-, physician-, and laboratory reported outcomes in recent-onset rheumatoid arthritis patients. Clin Exp Rheumatol. 2010;28:748–51.
Contreras- Yáñez I, Pascual-Ramos V. Predictors of health care dropout in an inception cohort of patients with early-onset rheumatoid arthritis. BMC Musculoskeletal Dis. 2017;18:1–9.
Pincus T, O’Dell JR, Kremer JM. Combination therapy with multiple disease-modifying antirheumatic drugs in rheumatoid arthritis: a preventive strategy. Ann Intern Med. 1999;131(10):768–74.
Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69(4):631–7.
Emery P, Salmon M. Early rheumatoid arthritis: time to aim for remission? Ann Rheum Dis. 1995;54(12):944–7.
Aletaha D, Ward MM, Machold KP, Nell VP, Stamm T, Smolen JS. Remission and active disease in rheumatoid arthritis: Defining criteria for disease activity states. Arthritis Rheum. 2005;52(9):2625-36. https://doi.org/10.1002/art.21235.
Gul H, Ferreira J, Emery P. Remission in rheumatoid arthritis: is it all the same? Expert Rev Clin Pharmacol. 2015;8:575–86.
Prevoo M, van’t Hof M, Kuper H, van Leeuwen M, van de Putte L, van Riel P. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8.
Mody G, Cardiel M. Challenges in the management of rheumatoid arthritis in developing countries. Best Pract Res Clin Rheumatol. 2008;22:621–41.
Massardo L, Pons-Estel B, Wojdyla D, Cardiel M, Galarza-Maldonado C, Furst DE, et al. Early rheumatoid arthritis in Latin America: low socioeconomic status related to high disease activity at baseline. Arthritis Care Res. 2012;64:1135–43.
Klerk E, van der Heijde D, Landewé R, van der Tempel H, Urquhart J, van der Linden S. Patient compliance in rheumatoid arthritis, polymyalgia rheumatica, and gout. J Rheumatol. 2003;30:44–54.
Deyo R, Inui T, Sullivan B. Noncompliance with arthritis drugs: magnitude, correlates, and clinical implications. J Rheumatol. 1981;8:931–6.
Tuncay R, Eksioglu E, Cakir B, Gurcay E, Cakci A. Factors affecting drug treatment compliance in patients with rheumatoid arthritis. Rheumatol Int. 2007;27:743–6.
Garcia-Gonzalez A, Richardson M, Popa-Lisseanu M, Cox V, Kallen M, Janssen N. Treatment adherence in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Rheumatol. 2008;27:883–9.
Lorish C, Richards B, Brown S. Perspective of the patient with rheumatoid arthritis on issues related to missed medication. Arthritis Care Res. 1990;3:78–84.
Grijalva C, Chung C, Arbogast P, Stein C, Mitchel E, Griffin M. Assessment of adherence to and persistence on disease-modifying antirheumatic drugs (DMARDs) in patients with rheumatoid arthritis. Med Care. 2007;45:66–76.
Curkendall S, Patel V, Gleeson M, Campbell R, Zagari M, Dubois R. Compliance with biologic therapies for rheumatoid arthritis: do patients out-of-pocket payments matter? Arthritis Rheum. 2008;59:1519–26.
Hoon L, Pullenayegu E, Moinedding R, Gladman D, Silverman E, Feldman B. Methods for analyzing observational longitudinal prognosis studies for rheumatic diseases: a review and worked example using a clinic-based cohort of juvenile dermatomyositis patients. Pediatr Rheumatol. 2017;29:15–8.
Cush J. Early rheumatoid arthritis – is there a window of opportunity? J Rheumatol. 2007;80 Suppl:1–7.
Contreras-Yáñez I, Pascual-Ramos V. Window of opportunity to achieve major outcomes in early rheumatoid arthritis patients: how persistence with therapy matters. Arthritis Res Ther. 2015;17:1–9.
Nell V, Machold K, Eberl G, Stamm T, Uffman M, Smolen J. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology. 2004;43:906–14.
Schett G, Emery P, Tanaka Y, Burmester G, Pisetsky D, et al. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann Rheum Dis. 2016;75:1428–37.
van den Broek M, Huizinga T, Dijkmans B, Allart C. Drug-free remission: is it already possible? Curr Opin Rheumatol. 2011;23:266–72.
Wabe N, Wiese M. Treating rheumatoid arthritis to target: physician and patient adherence issues in contemporary rheumatoid arthritis therapy. J Eval Clin Pract. 2017;23:486–93.
Seung J, Jung P, Sang-Wong L, Jungski J, Soo-Koon L, Yong-Beom P. Clinical characteristics associated with drug-free sustained remission in patients with rheumatoid arthritis: data from Korean Intensive Management of Early Rheumatoid Arthritis (KIMERA). Semin Arthritis Rheum. 2020;50:1414–20.
Kuijper T, Lamer-Karnebeek F, Jacobs J, Hazes J, Luime J. Flare rate pn Patients with rheumatoid arthritis in low disease activity or remission when tapering or stopping synthetic or biologic DMARD: a systematic review. J Rheumatol. 2015;42:2012–22.
O’Mahony R, Richards A, Deighton C, Scott D. Withdrawal of disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2010;69:1823–6.
Lee P, Tan LJ. Drug compliance in outpatients with rheumatoid arthritis. Aust NZ J Med. 1979;9(3):274–7.
Kuusalo L, Puolakka K, Kautiainen H, Eklund K, Ilva K, Kaipiainen-Seppänen O, et al. Impact of physicians’ adherence to treat-to-target strategy on outcomes in early rheumatoid arthritis in the NEO-RACo trial. Scand J Rheumatol. 2015;44:449–55.
Acknowledgements
None
Funding
None.
Author information
Authors and Affiliations
Contributions
ICY participated in the conception and design of the study, performed the statistical analysis, and substantively revised the manuscript. She is responsible for the integrity of the early RA cohort databases. GAGB participated in the conception, design, and analysis of the study and substantively revised the manuscript. MCM participated in the data acquisition of the study and creation of the figures in the manuscript. JJHB participated in the data acquisition of the study and creation of the figures in the manuscript. VPR participated in the conception, design, and analysis of the study; she is the clinician responsible for the early RA cohort and drafting of the manuscript. The authors read and approved the final manuscript.
Authors’ information
Not applicable
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán with the reference number IRE-274-10/11-1. All the patients provided written informed consent for clinical follow-ups when entering the clinic. They provided additional written permission to review each patient’s chart and present data in scientific publications.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Baseline population characteristics between patients who maintained DFR status and their counterparts.
Additional file 2: Table S2.
Matching criteria and percentage achieved in the controls’ selection.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Contreras-Yáñez, I., Guaracha-Basáñez, G.A., Cuevas-Montoya, M. et al. Early persistence on therapy impacts drug-free remission: a case-control study in a cohort of Hispanic patients with recent-onset rheumatoid arthritis. Arthritis Res Ther 24, 193 (2022). https://doi.org/10.1186/s13075-022-02884-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-022-02884-w