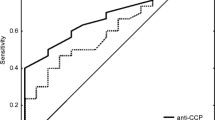
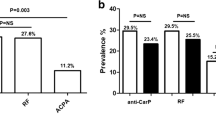
Abstract
Objectives
The aim of the study was to evaluate the frequency of anti-mutated citrullinated vimentin antibodies (a-Sa), anti-citrullinated α-enolase peptide 1 antibodies (a-CEP-1), anti-filaggrin antibodies (AFAs), heterogeneous nuclear ribonucleoprotein compies/anti-RA33-antibodies (a-hnRNP/RA33), anti-carbamylated protein antibodies (a-CarP), and metalloproteinase (MMPs) activity in patients with early inflammatory arthritis (EIA).
Methods
Seventy-four patients with EIA: 51 diagnosed with RA (rheumatoid arthritis) and 23 with UA (undifferentiated arthritis), and 20 healthy volunteers were enrolled to the study. Inflammatory markers, rheumatoid factor (RF), and antibodies mentioned above were assessed in all patients.
Results
In the EIA group, we observed significantly higher concentration of a-CEP-1 (65.8 ± 111.6 RU/mL) than in controls (2.0 ± 0.0 RU/mL). In RF(+) RA patients, we observed higher concentration of a-Sa and a-CEP-1 than in other groups. A-Sa were positive in 69% of RF(+) RA, 37% of RF(−) RA, 26% of UA patients and in 10% of controls. A–CEP-1 were positive in 77% of RF(+) RA patients, in 56% of RF(−) RA patients, in 8.7% of UA patients, but they were negative in controls. In patients with RF(+) RA, positive a-CarP were present statistically significantly more often than in RF (−) RA patients. No statistically significant difference in frequency of a-hnRNP/RA33 and AFA between RF(+) RA, RF(−) RA, and UA was observed.
Conclusions
Our results suggest that a-CEP-1 may help in differentiation between RF(−) RA and UA. a-CEP-1 and a-Sa may be useful while diagnosing EIA. a-CarP may be used in differentiation of RA RF(−) and UA. However, a follow-up study is needed to evaluate the prognostic value of analyzed antibodies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early inflammatory arthritis (EIA) is defined as inflammation of one or more joints lasting up to 6–12 weeks, or even up to 12 months according to some authors. In approx. 20–60% of patients, the disease has a self-limited course. Depending on the selection criteria, studies on EIA revealed that 13–54% of patients may progress to rheumatoid arthritis (RA). In other cases, another disease may be identified in follow-up, but the disease may also remain at the undifferentiated arthritis (UA) stage in 2–87% of patients, with no progression towards any of the typical arthropathies [1,2,3,4].
In terms of prognosis, it is extremely important to identify patients with increased risk of developing RA, as early initiation of disease-modifying treatment has been demonstrated to inhibit disease progression, increase the likelihood of remission, and prevent joint destruction and disability, besides being economically beneficial. The term “window of opportunity,” introduced in rheumatology, defines the limited time within the natural course of the disease where the most effective treatment is possible. If the window of opportunity is missed, long-term prognosis becomes worse, often regardless of the treatment method used. This necessitates a search for new and better diagnostic methods to identify early RA. New screening forms to assess the risk of progression from UA to RA and RA risk calculators are being developed [5, 6].
Besides imaging methods (ultrasound and magnetic resonance imaging), which are important in early diagnostics, immunodiagnostics are of vital importance [7,8,9,10]. The presence of the rheumatoid factor (RF) still has diagnostic value. The factor is found in approx. 60–80% of RA patients. Anti-cyclic citrullinated peptide (a-CCP) autoantibodies are another fundamental immune marker. These antibodies may appear years before the first arthritic symptoms, and often earlier than RF. They are found in 60% of early RA cases. Various studies estimate a-CCP2 test sensitivity at 48–80%, and its specificity—at 96–98% [11, 12].
As the earliest diagnosis possible is extremely important, other immune markers that may speed up diagnosing are needed [13]. Literature discusses a number of immune markers found in RA that are not routinely used in diagnostics.
We selected five autoantibodies and two metalloproteinases, to investigate how frequently they are found and how useful they may be in patients with EIA. We studied the occurrence of anti-citrullinated vimentin (a-Sa), anti-citrullinated α-enolase peptide 1 (a-CEP-1), anti-filaggrin (AFA), anti-heterogeneous nuclear ribonucleoprotein (a-hnRNP/RA33), and anti-carbamylated protein (a-CarP) antibodies, and evaluated metalloproteinase 3 and 9 (MMP3, MMP9) activity. Subsequently, we evaluated the utility of these parameters in routine diagnostics and their value for RA diagnosis in uncertain cases.
Patients and methods
Patients
Seventy-four DMARD (disease-modifying antirheumatic drug)-naïve patients with EIA, defined as inflammation of one or more joints lasting up to 12 months, were enrolled to the study. Based on the clinical and laboratory assessment, 51 patients were diagnosed with early RA and 23 with UA. Exclusion criteria were the use of DMARDs or glucocorticoids, active infection, cancers, and other autoimmune diseases. RA patients met the 2010 ACR/EULAR (Americaln College of Rheumatology/European League Against Rheumatism) criteria for RA classification. Patients with UA did not meet the criteria for any rheumatic disease. Twenty healthy volunteers matched for sex and age were used as controls.
In all patients, the following routine laboratory tests were done: inflammatory markers (ESR—erythrocyte sedimentation rate, C-reactive protein—CRP), RF, and a-CCP. The tests were performed in a certified laboratory at the University Hospital, using commercially available test kits.
Immunological tests
Blood samples obtained from the patients were centrifuged at 3500 RPM and isolated serum was frozen at − 25 °C until it was used for the immune testing.
Serum levels of a-Sa, a-CEP-1, a-CarP, a-hnRNP/RA33, AFA, MMP3, and MMP9 were determined with commercial ELISA kits (anti-Sa ELISA(IgG) kit, EUROIMMUN Medizinische Labordiadnostica AG, Lubeck, Germany; anti CEP-1 ELISA(IgG) kit, EUROIMMUN Medizinische Labordiadnostica AG, Lubeck, Germany; a-Car-P-Human Anti-carbamylated-FCS ELISA kit, Sincere Biotech Co, Beijing, China; hnRNP/Ra-33-Human heterogeneous nuclear ribonucleoprotein complex/anti-RA-33-antibody ELISA kit MyBioSource, San Diego, USA; AFA-Human Filaggrin Antibody ELISA kit, Abbexa, Cambridge, UK; MMP-3-human matrix metalloproteinase-3 Platinum ELISA kit, eBioscience, San Diego, USA; MMP-9- human matrix metalloproteinase-9 Platinum ELISA kit, eBioscience, San Diego, USA, respectively) accordingly to the manufacture instruction.
Positive outcomes for the immune marker tests were defined as follows:
-
RF: levels > 14 IU/mL; high levels were defined as values exceeding the upper normal limit 3-fold or more
-
a-CCP: level > 5.0 U/mL; high levels were defined as values exceeding the upper normal limit 3-fold or more
-
a-Sa: level > 20 RU/mL
-
a-CEP-1: antibody count > 20 RU/mL
-
a-CarP: the mean from the control group (+ 2 SD) was used as the cut-off value (4.2 U/mL)
-
a-hnRNP/RA33: the mean from the control group (+ 2 SD) was used as the cut-off value (1.8 ng/mL)
-
AFA: the mean from the control group (+ 2 SD) was used as the cut-off value (14.9 ng/mL)
-
MMP3: activity increased > 28 ng/mL
-
MMP9: activity increased > 139.4 ng/mL
Assessment of disease activity
Disease activity was evaluated using the scale recommended by EULAR—DAS28 (Disease Activity Score 28). The DAS28 result comprises the following variables: tender joint count (TJC), swollen joint count (SJC) out of 28, ESR or CRP, and an overall disease activity evaluation provided by the patient using a 100-mm visual analog scale (VAS).
Ethical statement
The study was performed in accordance with the Helsinki Declaration of 1975, as amended in 2000, and the locally applicable laws. It was approved by the Bioethics Committee of the Wrocław Medical University, approval no. 469/2010.
Statistics
Quantitative variables are expressed as means and standard deviation (SD). Qualitative variables are expressed as frequency and percentage. The statistical analysis were carried out using STATISTICA software (data analysis software system), version 12 (StatSoft, Inc., Tulsa, OK, USA). Student’s t test for independent samples was used for comparison in means between two groups while a one-way ANOVA with NIR post hoc test was performed for comparison involving three or more groups. Comparison in proportions between groups was performed with chi-square (or Fisher exact test when required). All analyses were performed two-tailed and the limit of significance was set at p < 0.05.
Results
Seventy-four patients (73% females) with EIA and 20 healthy controls were enrolled to the study. Mean patients’ age was 48.5 years (range: 18–85 years). Sixty-nine percent (51) of the patients were diagnosed with RA and 31% (23) with UA.
Mean duration of joint swelling before inclusion to the study was 5.5 months (range 1–12 months). No difference between analyzed subgroups in arthritis duration and disease activity was found.
Epidemiological and clinical data of EIA patients are shown in Table 1.
In RA group, RF was detected in 35 (68.6%) patients and a-CCP in 34 (66.7%) patients. Among RF (+) patients, high level of RF was revealed in 68% of them. On the other hand, high a-CCP level was detected in 85% of a-CCP (+) patients.
Seventy-five percent of patients with RA RF(−) were also negative for a-CCP antibodies. Ninety-one percent of UA patients were negative both for a-CCP and f RF. An analysis of RF test results from all patients showed a sensitivity of 68.6% and a specificity of 94.6%. In the same group, a sensitivity of 66.7% and specificity of 97.3% was found for the a-CCP antibody test.
Autoantibodies
The distribution of serological marker levels (a-Sa, a-CEP-1, AFA, a-CarP, a-hnRNP/RA33, MMP3, MMP9) for all patients with early arthritis and controls is shown in Table 2.
Patients with EIA had significantly higher CEP-1 levels (65.8 ± 111.6 RU/mL) than healthy controls (2.0 ± 0.0 RU/mL).
The a-Sa antibody test was positive in 69% of patients with RF(+) RA, 37% with RF(−) RA, 26% with UA, and 10% healthy controls. Eight patients negative for both a-CCP and RF were positive for a-Sa—three of them were diagnosed with RF(−) RA and five with UA. The a-Sa antibody test was positive in 18.8% of RA patients negative for a-CCP and RF and in 21.7% of UA patients negative for a-CCP and RF. The incidence of a-Sa positivity was significantly higher in EIA (47.3%) than in healthy controls and in RA (56.8%) than in UA.
The CEP-1 antibody test was positive in 77% of patients with RA RF(+), 56% with RA RF(−), 8.7% with UA, and negative in all controls. Prevalence of a-CEP-1 in RA patients was significantly higher than in healthy controls and UA patients. Seven patients negative for both a-CCP and RF were positive for CEP-1 antibodies—six of them were diagnosed with RA RF(−) and one with UA. Therefore, the CEP-1 antibody test was positive in 50% of RA RF(−) patients negative for a-CCP and RF and in just 4.5% of UA patients negative for a-CCP and RF.
The a-CarP test was positive in 40% of RF(+) RA patients, 6.3% of RF(−) RA patients, and 21.7% of UA patients. Patients with RF(+) RA were positive for a-CarP significantly more often than patients with RF(−) RA. No differences were found for the remaining groups.
The a-hnRNP/RA33 test was positive in 45.7% of RF(+) RA patients, 18.8% of RF(−) RA patients, and 30.4% of UA patients; however, the difference was statistically insignificant.
The AFA test was positive in 25.7% of RF(+) RA patients, 18.8% of RF(−) RA patients, and 17.4% of UA patients; however, the differences did not reach statistical significance.
In EIA patients, no correlation between smoking and a-Sa, CEP-1, a-CarP, a-hnRNP/RA33, AFA, MMP3, or MMP9 levels was found. Patients who smoked had significantly higher CRP and ESR values.
Discussion
The presence of autoantibodies (RF and a-CCP) is an important predictive factor for RA development [13]. The a-CCP-positive healthy relatives of RA patients have a positive predictive value for RA development in 61% of cases, compared to 0.4% among a-CCP-negative relatives. Seropositive individuals with joint-related symptoms or arthralgia are at a 50% risk of developing RA within a year [14, 15]. The immune marker values reported in the present paper are typical for the RA population, and the sensitivity and specificity of RF and a-CCP tests in RA patients are comparable with literature data [16].
There is an ongoing search for other biomarkers that would improve RA diagnosis, allow the assessment of the risk of rapid progression, and help to verify the efficacy of the treatment used.
Beside a-CCP antibodies, AFA, a-CEP-1, and a-Sa antibodies may also be formed in the citrullination process. Some authors reported that A-CEP-1 antibodies are found in approx. 25–40% of patients with early RA [17]. They have high specificity (approx.. 98%), and their level decreases during treatment. Their presence may be associated with genetic and environmental factors, such as smoking and periodontal disease [18]. Based on the study performed, we demonstrated that patients with EIA had higher a-CEP-1 antibody levels than healthy controls. Moreover, RA patients positive for RF had significantly higher a-CEP-1 antibody levels. Besides this, in our study prevalence of a-CEP-1 was significantly higher in RA patients comparing to UA patients. Contrary to the study by Fisher et al., we found no correlation between the presence of a-CEP-1 and smoking [19]. These authors found positive a-CCP and a-CEP-1 tests in patients previously diagnosed with RA to be strongly correlated with smoking and with the HLA-DRB1 genetic profile. The difference in findings may be associated with the short duration of disease in the patients included in our study. Fisher et al. reported no differences in the clinical course of the disease or response to DMARDs in patients a-CCP (+) who were either positive or negative for a-CEP-1. In their study, two RA patient cohorts were evaluated—Karolinska and NOAR (Norfolk Arthritis Register). In Karolinska cohort, 57% of patients were positive for a-CCP and 27% were positive for a-CEP-1; in NOAR, 50% were positive for a-CCP and 24% for CEP-1. In both groups, most patients positive for a-CEP-1 were positive for a-CCP (92 and 85%). In our study, 85.3% of RA a-CCP (+) patients were a-CEP-1 (+), while 80.6% of patients positive for a-CEP-1 were positive for a-CCP. This indicates a strong correlation between the presence of CEP-1 and a-CCP antibodies. The increased occurrence of a-CCP antibodies in RA a-CEP-1(+) patients was also reported by Montes et al. [20]. In that study, patients positive for both markers had a significantly higher frequency of the DRB1 genotype and a more rapid radiographic progression. An association of a-CEP-1 antibody presence with smoking, as well as with the presence of the genetic risk factor for RA (the HLA-DRB1 shared epitope and PTPN22), was also reported by Mahdi et al. [21]. Alunno et al. reported association between occurrence of a-CEP-1 and erosive RA [17]. Other studies did not confirm the differences in clinical course and response to treatment associated with the concurrent presence of these two types of antibodies [19].
A-Sa antibodies target the protein component of rheumatoid pannus (vimentin). In RA, they are present in 31–44% of patients, less commonly at the early stages of the disease. They are distinguished themselves by their high specificity, 92–98% [22].
Our analysis demonstrated that patients with RF(+) early RA have also higher levels of a-Sa antibodies. Therefore, the potential for broader use of these parameters in routine arthritis diagnostics should be considered, particularly since some literature data confirm also their added prognostic value. The presence of a-Sa antibodies may help to identify a subset of RA patient with aggressive early erosive disease [23]. Both markers have a high specificity and sensitivity, also corroborated in our study [22,23,24].
The presence of a-Sa antibodies in RA RF (+) is discussed by Safi et al., who conclude that the two markers are correlated [25]. In his study, it is also shown that high ESR and CRP values correlated with RF positivity but not with the a-Sa positivity. Additionally, Qu et al. tested RF(−) RA patients for a-Sa in the synovial fluid [26]. Their results suggest that these tests may be useful in differential diagnosis for seronegative RA and osteoarthritis. A study by Zahran et al. confirms also the utility of testing for a-Sa. In the study, 33% of RA a-CCP (−) patients and approx. 40% of RA RF (−) patients were found to have increased levels of these antibodies. Additionally, the presence of a-Sa was found to be positively correlated with ESR and CRP [27]. It is suggested that a-Sa level is a better indicator of radiographic progression risk than a-CCP level [28]. The authors of these studies even suggest that these antibody tests should be included as an additional point in the 2010 ACR/EULAR criteria for RA classification when RF and a-CCP antibodies are negative. The benefits of the a-Sa antibody test in RA patients are also corroborated by Yang Fen Hou [29]. Iwaszkiewicz et al. reached a different conclusion. They found no added diagnostic value from a-Sa testing, as RF (−) patients were also a-Sa (−) [22]. In our study, 25% of RF(−) RA and 22% of UA patients a-Sa (+). Follow-up observation of these patients in the next several years is important. In a study by Chalenger et al., a-Sa and a-CarP antibodies often co-occurred [30].
Monts et al. analyzed the serological profile of RA patients in terms of a-CEP-1 and a-Sa antibodies [31]. Only patients positive for both a-CCP and a-Sa antibodies were at a higher risk of radiographic progression, and patients positive for a-CCP and a-CEP-1 were not. These results are, however, denied with other studies [32].
In our study, testing for a-Sa antibodies in patients with RF(−) RA and UA did not contribute to differentiation between the two diseases. a-CEP-1 antibody testing in the same group offered a higher likelihood of differentiation between seronegative RA and UA, though this finding requires further studies.
Most studies involved patients already diagnosed with RA. Moreover, the concurrent presence of both antibodies with a-CCP is emphasized. In our study, however, there were patients positive for a-Sa and a-CEP-1 antibodies and negative for a-CCP. This warrants further research involving larger groups of patients, as such testing could potentially assist in diagnosing the so-called seronegative RA.
AFA antibodies are found in 40% of RA patients. They target the protein component of the cytoskeleton filaments (filaggrin) and are involved in epidermal keratinization. According to the available literature, they are found in up to 45% of patients with the so-called seronegative RA. Their specificity is estimated at 99%, but the sensitivity may be as low as 50% [32]. The utility of AFA antibodies as a marker of very early arthritis was demonstrated by Vittecoq et al. [33]. The authors found that AFA co-occurred with RF in patients with arthritis who did not meet RA diagnosis criteria at first evaluation. The diagnosis was then made at follow-up. In our group of arthritis patients who did not meet RA classification criteria, AFA antibodies were found in 17.4% of patients. Further observation is required to definitively determine the utility of AFA testing for the patient group studied. Based on our study, testing for MMP3 activity may be significant for differentiating between UA and seronegative RA. We found that MMP3 levels were significantly higher in seronegative RA than in UA. Similar data were reported by Hiura et al. in their study. The authors found that the combination of MMP3 activity, CRP levels, and positive RF was associated with a higher likelihood of RA diagnosis after a 12-month follow-up in a-CCP (−) patients initially diagnosed with UA [34]. Hattori et al. emphasized the association between MMP3 activity, CRP levels, and disease activity [35]. Other studies also demonstrate the role of MMP3 in disease activity evaluation and confirm that MMP3 activity is increased in RA patients [36, 37].
a-CarP and a-hnRNP/RA33 antibodies are immune markers produced in another mechanism. In carbamylation, the lysine amino acid is transformed into homocitrullin. a-CarP antibodies are found in approx. 40% of RA patients. They may be present in the serum years before the occurrence of arthritic symptoms, and according to some authors, they are correlated with radiographic progression [11, 38,39,40]. They occur both in seropositive and seronegative RA. A-hnRNP/RA33 antibodies are found in a range of autoimmune rheumatic diseases. They react with pre-mRNA and are involved in cellular processes such as DNA repair and chromatin remodeling. In early RA, they are found in 14% of cases [41]. In patients diagnosed with RA, they have high sensitivity, up to 98%, but low specificity (20%), according to literature reports [42].
With regard to a-CarP antibody testing in patients with early RA, Requeiro et al. concluded that its utility in RA diagnosis is dubious, though the testing may be beneficial in seronegative patients [43]. The prognostic value of these markers was not precluded. Similarly, in our study, besides the significantly more common presence of a-CarP antibodies in RF(+) than in RF(−) RA, the antibodies were also found in 21% of patients with UA, which might facilitate diagnosis in uncertain cases. Another study suggesting an association between a-CarP and faster radiographic progression, independent of a-CCP, was published by Brink et al. [44]. The authors also emphasize the fact that these antibodies may be found in patients long before RA is diagnosed.
In our study, a-RA33 antibodies were found in some patients with RA RF(−) and UA. The utility of such testing in EIA with no classical serological markers was suggested by Lashkari et al. [42]. However, the diagnostic value of these antibodies is diminished by their occurrence in other autoimmune diseases [41]. To sum up, our results suggest that a-CEP-1, a-CarP, and MMP 3 may help in differentiation between RF(−) RA and UA. Besides this, a-CEP-1 and a-Sa may be useful while diagnosing EIA. However, a follow-up study is needed to evaluate the prognostic value of analyzed antibodies.
References
Norli ES, Brinkmann GH, Kvien TK, Bjørneboe O, Haugen AJ, Nygaard H et al (2017) Diagnostic spectrum and 2-year outcome in a cohort of patients with very early arthritis. RMD Open [Internet]. [cited 2018 Apr 30];3:e000573. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29299343
Hua C, Daien CI, Combe B, Landewe R (2017) Diagnosis, prognosis and classification of early arthritis: results of a systematic review informing the 2016 update of the EULAR recommendations for the management of early arthritis. RMD Open 3:1–10
Söderlin MK, Börjesson O, Kautiainen H, Skogh T, Leirisalo-Repo M (2002) Annual incidence of inflammatory joint diseases in a population based study in southern Sweden. Ann Rheum Dis 61:911–915
Hazes JMW, Luime JJ (2011) The epidemiology of early inflammatory arthritis. Nat Rev Rheumatol 7:381–390 Available from: http://www.nature.com/doifinder/10.1038/nrrheum.2011.78
van der Helm-van Mil AH, le Cessie S, van Dongen H, Breedveld FC, Toes RE, Huizinga TW (2007) A prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: how to guide individual treatment decisions. Arthritis Rheum 56:433–440
Van De Stadt LA, Witte BI, Bos WH, Van Schaardenburg D (2013) A prediction rule for the development of arthritis in seropositive arthralgia patients. Ann Rheum Dis 72:1920–1926
Naredo E, Collado P, Cruz A, Palop MJ, Cabero F, Richi P, Carmona L, Crespo M (2007) Longitudinal power Doppler ultrasonographic assessment of joint inflammatory activity in early rheumatoid arthritis: predictive value in disease activity and radiologic progression. Arthritis Care Res 57:116–124
Østergaard M, Pedersen SJ, Døhn UM (2008) Imaging in rheumatoid arthritis—status and recent advances for magnetic resonance imaging, ultrasonography, computed tomography and conventional radiography. Best Pract Res Clin Rheumatol 22:1019–1044
Kawashiri SY, Suzuki T, Okada A, Yamasaki S, Tamai M, Nakamura H, Origuchi T, Mizokami A, Uetani M, Aoyagi K, Eguchi K, Kawakami A (2013) Musculoskeletal ultrasonography assists the diagnostic performance of the 2010 classification criteria for rheumatoid arthritis. Mod Rheumatol 23:36–43
Wakefield RJ, Gibbon WW, Conaghan PG, O’Connor P, McGonagle D, Pease C et al (2000) The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: a comparison with conventional radiography. Arthritis Rheum 43:2762–2770
Shi J, van de Stadt LA, Levarht EWN, Huizinga TWJ, Hamann D, van Schaardenburg D, Toes REM, Trouw LA (2014) Anti-carbamylated protein (anti-CarP) antibodies precede the onset of rheumatoid arthritis. Ann Rheum Dis 73:780–783
Scott DL, Wolfe F, Huizinga TWJ (2010) Rheumatoid arthritis. Lancet 376:1094–1108
Combe B, Landewe R, Daien CI, Hua C, Aletaha D, Álvaro-Gracia JM et al (2017) 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis [Internet]. [cited 2018 May 2];76:948–59. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27979873
Ramos-Remus C, Castillo-Ortiz JD, Aguilar-Lozano L, Padilla-Ibarra J, Sandoval-Castro C, Vargas-Serafin CO, de la Mora-Molina H, Ramos-Gomez A, Sanchez-Ortiz A, Avila-Armengol H, Aceves-Avila FJ (2015) Autoantibodies in prediction of the development of rheumatoid arthritis among healthy relatives of patients with the disease. Arthritis Rheum 67:2837–2844
Rakieh C, Nam JL, Hunt L, EMA H, Das S, Bissell LA et al (2015) Predicting the development of clinical arthritis in anti-CCP positive individuals with non-specific musculoskeletal symptoms: a prospective observational cohort study. Ann Rheum Dis 74:1659–1666
Matuszewska A, Madej M, Wiland P (2016) Immunological markers of rheumatoid arthritis. Postepy Hig Med Dosw 70:251–257
Alunno A, Bistoni O, Pratesi F, La Paglia GMC, Puxeddu I, Migliorini P et al (2018) Anti-citrullinated alpha enolase antibodies, interstitial lung disease and bone erosion in rheumatoid arthritis. Rheumatology [Internet]. [cited 2018 May 2];57:850–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29452423
Bialowas K, Swierkot J, Radwan-Oczko M (2014) Role of Porphyromonas gingivalis in rheumatoid arthritis and inflammatory spondyloarthropathies. Postepy Hig Med Dosw (Online) 68:1171–1179
Fisher BA, Plant D, Brode M, van Vollenhoven RF, Mathsson L, Symmons D et al (2011) Antibodies to citrullinated α-enolase peptide 1 and clinical and radiological outcomes in rheumatoid arthritis. Ann Rheum Dis 70:1095–1098
Montes A, Dieguez-Gonzalez R, Perez-Pampin E, Calaza M, Mera-Varela A, Gomez-Reino JJ, Gonzalez A (2011) Particular association of clinical and genetic features with autoimmunity to citrullinated α-enolase in rheumatoid arthritis. Arthritis Rheum 63:654–661
Mahdi H, Fisher BA, Källberg H, Plant D, Malmström V, Rönnelid J, Charles P, Ding B, Alfredsson L, Padyukov L, Symmons DPM, Venables PJ, Klareskog L, Lundberg K (2009) Specific interaction between genotype, smoking and autoimmunity to citrullinated α-enolase in the etiology of rheumatoid arthritis. Nat Genet 41:1319–1324
Iwaszkiewicz C, Puszczewicz M, Bialkowska-Puszczewicz G (2015) Diagnostic value of the anti-Sa antibody compared with the anti-cyclic citrullinated peptide antibody in rheumatoid arthritis. Int J Rheum Dis 18:46–51
Mansour HE, Metwaly KM, Hassan IA, Elshamy HAA, Elbeblawy MMS (2010) Antibodies to mutated citrullinated vimentin in rheumatoid arthritis: diagnostic value, association with radiological damage and axial skeleton affection. Clin Med Insights Arthritis Musculoskelet Disord 3:33–42
Vossenaar ER, Després N, Lapointe E, van der Heijden A, Lora M, Senshu T, van Venrooij WJ, Ménard HA (2004) Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res Ther 6:R142–R150
Safi MAA, Attar SM, Fathaldin OA, Safi OMA (2015) Anti-mutated citrullinated vimentin antibody and rheumatoid factor (prevalence and association) in rheumatoid arthritis patients; Saudi and non-Saudi. Clin Lab 61:259–267
Qu SJ, Ye H, Jia RL, Li ZG (2016) Significance and diagnostic value of synovial fluid anti-cyclic citrullinated peptide antibody and anti-mutated citrullinated vimentin antibodies in patients with serum negative rheumatoid arthritis. Beijing Da Xue Xue Bao 48:933–936
Zahran WE, Mahmoud MI, Shalaby KA, Abbas MH (2013) Unique correlation between mutated citrullinated vimentine IgG autoantibodies and markers of systemic inflammation in rheumatoid arthritis patients. Indian J Clin Biochem 28:272–276
Keskin G, Inal A, Keskin D, Pekel A, Baysal O, Dizer USA (2008) Diagnostic utility of anti-cyclic citrullinated peptide and anti-modified citrullinated vimentin antibodies in rheumatoid arthritis. Protein Pept Lett 15:314–317
Hou YF, Sun GZ, Sun HS, Pan WP, Liu WB, Zhang CQ (2012) Diagnostic value of anti-Sa and anticitrullinated protein antibodies in rheumatoid arthritis. J Rheumatol 39:1506–1508
Challener GJ, Jones JD, Pelzek AJ, Hamilton BJ, Boire G, De Brum-Fernandes AJ et al (2016) Anti-carbamylated protein antibody levels correlate with anti-sa (citrullinated vimentin) antibody levels in rheumatoid arthritis. J Rheumatol 43:273–281
Montes A, Perez-Pampin E, Calaza M, Gomez-Reino JJ, Gonzalez A (2012) Association of anti-citrullinated vimentin and anti-citrullinated α-enolase antibodies with subsets of rheumatoid arthritis. Arthritis Rheum 64:3102–3110
Cantagrel A, Degboé Y (2016) New autoantibodies associated with rheumatoid arthritis recognize posttranslationally modified self-proteins. Joint Bone Spine 83:11–17
Vittecoq O, Jouen-Beades F, Krzanowska K, Bichon-Tauvel I, Ménard JF, Daragon A et al (2001) Rheumatoid factors, anti-filaggrin antibodies, and low in vitro interleukin 2 and interferonγ production are useful immunological markers for early diagnosis of community cases of rheumatoid arthritis. Preliminary study. Rev du Rhum (Edition Fr) 68:239–49
Hiura K, Iwaki-Egawa S, Kawashima T, Fujisawa SI, Takeda T, Komori H, Watanabe Y (2013) The diagnostic utility of matrix metalloproteinase-3 and high-sensitivity C-reactive protein for predicting rheumatoid arthritis in anti-cyclic citrullinated peptide antibody-negative patients with recent-onset undifferentiated arthritis. Rheumatol Int 33:2309–2314
Hattori Y, Kida D, Kaneko A (2017) Normal serum matrix metalloproteinase-3 levels can be used to predict clinical remission and normal physical function in patients with rheumatoid arthritis. Clin Rheumatol. https://doi.org/10.1007/s10067-017-3829-9
Zhou L, Song J, Chen L, Xu HJ (2016) The application of matrix metalloproteinase-3 and 7 joints ultrasonic score in assessment of disease activity in patients with rheumatoid arthritis. Zhonghua Nei Ke Za Zhi 55:531–534
Fiedorczyk M, Klimiuk PA, Sierakowski S, Gindzienska-Sieskiewicz E, Chwiecko J (2006) Serum matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with early rheumatoid arthritis. J Rheumatol 33:1523–1529
Gavrilă BI, Ciofu C, Stoica V (2016) Biomarkers in rheumatoid arthritis, what is new? J Med Life 9:144–148
Montes A, Regueiro C, Perez-Pampin E, Boveda MD, Gomez-Reino JJ, Gonzalez A (2016) Anti-carbamylated protein antibodies as a reproducible independent type of rheumatoid arthritis autoantibodies. PLoS One 11:e0161141
Stoop JN, Liu BS, Shi J, Jansen DTSL, Hegen M, Huizinga TWJ, Trouw LA, Toes REM (2014) Antibodies specific for carbamylated proteins precede the onset of clinical symptoms in mice with collagen induced arthritis. PLoS One 9:e102163
Maslyanskiy A, Lazareva N, Olinek P, Schierack P, Hentschel C, Cuccato J, Bogdanos DP, Lapin SV, Roggenbuck D (2014) Anti-hnRNP B1 (RA33) autoantibodies are associated with the clinical phenotype in Russian patients with rheumatoid arthritis and systemic sclerosis. J Immunol Res 2014:1–7
Lashkari M, Noori A, Hajiimanouchehri F, Oveisi S, Kazemifar AM (2014) Determination of specificity and sensitivity of anti-RA 33 in diagnosis of early rheumatoid arthritis. Global J Health Sci 6:292–297
Regueiro C, Nuño L, Ortiz AM, Peiteado D, Villalba A, Pascual-Salcedo D, Martínez-Feito A, González-Alvaro I, Balsa A, González A (2017) Value of measuring anti-carbamylated protein antibodies for classification on early arthritis patients. Sci Rep 7:12023
Brink M, Verheul MK, Rönnelid J, Berglin E, Holmdahl R, Toes REM, Klareskog L, Trouw LA, Rantapää-Dahlqvist S (2015) Anti-carbamylated protein antibodies in the pre-symptomatic phase of rheumatoid arthritis, their relationship with multiple anti-citrulline peptide antibodies and association with radiological damage. Arthritis Res Ther 17:25
Acknowledgments
This study was supported by Wroclaw Medical University ST-925.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was performed in accordance with the Helsinki Declaration of 1975, as amended in 2000, and the locally applicable laws. It was approved by the Bioethics Committee of the Wrocław Medical University, approval no. 469/2010.
Disclosures
None.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ponikowska, M., Świerkot, J., Nowak, B. et al. Autoantibody and metalloproteinase activity in early arthritis. Clin Rheumatol 38, 827–834 (2019). https://doi.org/10.1007/s10067-018-4326-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-018-4326-5
Keywords
- Anti-carbamylated protein antibodies (a-CarP)
- Anti-citrullinated α-enolase peptide 1 antibodies (a-CEP-1)
- Anti-filaggrin antibodies (AFA)
- Anti-mutated citrullinated vimentin antibodies (a-Sa)
- Early inflammatory arthritis
- Heterogeneous nuclear ribonucleoprotein compies/anti-RA33-antibodies (a-hnRNP/RA33)
- Metalloproteinases (MMP)
- Rheumatoid arthritis