Abstract
Extracorporeal CO2 removal (ECCO2R) is intended to facilitate lung protective ventilation in patients with hypercarbia. The combination of continuous renal replacement therapy (CRRT) and minimal-flow ECCO2R offers a promising concept for patients in need of both. We hypothecated that this system is able to remove enough CO2 to facilitate lung protective ventilation in mechanically ventilated patients. In 11 ventilated patients with acute renal failure who received either pre- or postdilution CRRT, minimal-flow ECCO2R was added to the circuit. During 6 h of combined therapy, CO2 removal and its effect on facilitation of lung-protective mechanical ventilation were assessed. Ventilatory settings were kept in assisted or pressure-controlled mode allowing spontaneous breathing. With minimal-flow ECCO2R significant decreases in minute ventilation, tidal volume and paCO2 were found after one and three but not after 6 h of therapy. Nevertheless, no significant reduction in applied force was found at any time during combined therapy. CO2 removal was 20.73 ml CO2/min and comparable between pre- and postdilution CRRT. Minimal-flow ECCO2R in combination with CRRT is sufficient to reduce surrogates for lung-protective mechanical ventilation but was not sufficient to significantly reduce force applied to the lung. Causative might be the absolute amount of CO2 removal of only about 10% of resting CO2 production in an adult as we found. The benefit of applying minimal flow ECCO2R in an uncontrolled setting of mechanical ventilation might be limited.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ventilation with low tidal volumes of 6–8 ml/kg ideal body weight (IBW) has become standard of care in ARDS patients [1]. This concept seemingly helps to decrease the risk of baro- and volutrauma on the affected lung and apparently even affects outcome favorably [2]. But today these principles are challenged: an IBW-based low Vt-strategy might not solely be sufficient to avoid tidal hyperinflation [3, 4]. Derived from these principles the concept of ultraprotective ventilation has been developed [5, 6]. However, a recently introduced concept stresses that no single variable (e.g. tidal volume or applied plateau- or delta pressure), but the mechanical energy inflicted on the lung corresponds to VILI (ventilator induced lung injury) [7]. The inevitable risk of consecutive hypercarbia might be accepted to a certain amount, but the effects of hypercarbia on whole-body physiology including the lung itself should not be neglected. Nevertheless, it has been shown that CO2 may also have a beneficial effect [8]. Side effects of hypercarbia include academia, increased shunt, shifted oxyhemoglobin dissociation curve [9], but also cardiac depression and impaired coronary artery perfusion [10]. As a consequence, further Vt-reduction might require additional extracorporeal support or dead space reductions to cope with these effects [4, 11,12,13].
The concept of extracorporeal CO2 removal was developed decades ago in the 1970s [14], but it required the growing knowledge on mechanisms of ventilator induced lung injury, recent technical developments and advanced options of dealing with complications [15, 16] to finally become clinically useful. Nowadays, different ECLA-devices (extracorporeal lung assist) are in clinical use. Other than active high-flow devices (extracorporeal membrane oxygenation, ECMO), mid-flow (about 1 l/min) and low-flow (about 500 ml/min) devices are readily used. Additionally, passive devices utilizing arteriovenous pressure difference are used.
In critically ill patients ARDS and renal failure (ARF) are commonly present simultaneously [17] and mortality remains unacceptably high [18]. Thus, both continuous renal replacement therapy (CRRT) and extracorporeal CO2-elimination can be necessary. With a large bore catheter for CRRT in place, allowing for higher flow rates than regular multi-lumen catheters the mental leap to add CO2-removal is obvious. Difficulties arise, because CO2-removal is flow dependent. Different concepts have been strived to facilitate CO2 removal in low flow rates, some of them based on the Henderson–Hasselbalch-equation to convert CO2/H + to bicarbonate, which is then dialyzed [19,20,21,22,23,24,25,26,27,28,29,30,31], others on electrical dialysis [32]. But none of these concepts has made the transition to clinical usefulness.
In this light, a device integrating both processes into one single extracorporeal circuit (CRRT-ECCO2R) offers potential advantages. Although the low blood flow that is applied during CRRT cannot provide sufficient oxygenation and only limited CO2 elimination, it might be enough to facilitate lung protective mechanical ventilation [13]. Based on this rational we aimed to test, whether minimal-flow CRRT-ECCO2R allows for a viable increase in lung protective ventilation without the undesirable side effects of hypercarbia or respiratory acidosis.
During standardized conditions and application of volume-controlled mechanical ventilation the potential to reduce Vt by addition of ECCO2R has been shown [13]. The effect of ECCO2R during pressure controlled or pressure support ventilation, that allows or assists spontaneous breathing are less defined and might vary. We purposely did not control for the ventilator mode and settings applied, moreover treating physicians were blinded to whether CO2-removal was active or not and ventilator settings were left to their judgment.
We hypothecated that a minimal-flow CRRT-ECCO2R is able to remove enough CO2 to facilitate lung protective ventilation in mechanically ventilated patients.
Methods
The local ethics committee approved the study prior to patient enrollment. We included adult patients (age > 18 years) in need for mechanical ventilation and CRRT; hypercarbia was considered neither inclusion nor exclusion criterion. Patients were not eligible for the study if they were hemodynamically unstable (doses of norepinephrine or epinephrine ≥ 0.1 µg/kg/min), if they were in severe acute respiratory distress (PaO2/FiO2 < 100), if they were thrombopenic (platelets < 50,000/µl), if they had an acute bleeding (> 200 ml/h) or if further therapy was futile due to bad prognosis or patients will. Assessed patient characteristics consisted of age, sex, weight, height, diagnoses, SAPS II score, ICU-stay, ventilator times, and duration of renal replacement therapy.
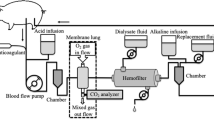
For this trial we used a combined CRRT-ECCO2R device (EQUA-smart®, Hemodec (MEDIA), Modena; Italy), basic settings were employed following manufacturer guidelines.
Ventilation was conducted using conventional ICU ventilators (Evita 4, Dräger, Lübeck, Germany or Servo-i, Maquet Critical Care, Solna Sweden). Peak inspiratory pressure (Pinsp) was limited to 30 ccmH2OO, ventilation modes used were either biphasic positive inspiratory airway pressure ventilation (Evita 4: BiPAP mode, Servo-i: BIVENT) or pressure support ventilation, depending on individual patient needs. ABGs were conducted frequently and used to modify ventilator settings aiming to keep paCO2 and pH as constant as possible during the preparatory phase of the trial.
CRRT was conducted using the CVVHDF mode, extraction dose 35 ml/kg, blood flow 300 ml/min, O2-flow 10 l/min, and all parameters were kept constant throughout. Patients were randomized to receive either pre- or postdilution.
CRRT was started as indicated without CO2 elimination. Then CO2 elimination was added and closely monitored for 6 h keeping blood- and gas flow constant throughout the study period. After the course of 6 h of CRRT plus ECCO2R we switched back to CRRT only. Treating physicians were blinded to the phase of the trial, i.e. if CO2 removal was active or just CRRT. The following parameters were collected just before starting CO2-removal as well as 1, 3, and 6 h during ECCO2R: arterial blood pressure, heart rate, mode of ventilation, respiratory rate, tidal volume, FiO2, PEEP, peak inspiratory pressure (Pinsp), and ABG (paO2, paCO2, pH, SaO2). Data were collected using the standard PDMS system in the unit (ICCA®, Philipps) and the integrated data recorder of the EQUAsmart® device. Data were extracted from both sources and stored in a Microsoft® Excel® Worksheet.
We analyzed changes in minute ventilation, tidal volume, respiratory rate, power applied to the lung, and paCO2 as well as CO2 removal.
Furthermore, we compared CO2 removal using pre- vs. postdilution. To quantify CO2 removal, we calculated the difference in CO2 contained in the afferent and efferent limb of the circuit. The calculation was performed using the equation described by Douglas et al. [33].
All parameters were tested for normal distribution using the Kolmogorov–Smirnov test, nevertheless due to small sample size we used statistical methods not reliant on normal distribution. Parameters were tested using the Kendall-test first and, if significance was found, additionally with the Wilcoxon-test for linked samples, we only report the Wilcoxon p value unless otherwise stated. CO2 removal pre- vs. postdilution was tested using Mann–Whitney-U-test for unrelated samples. Statistical calculations were done utilizing SPSS® Software Version 24, level of significance was assumed to be p < 0.05.
Results
We enrolled 14 patients, 3 of them had to be excluded from analysis due to incomplete datasets, leaving 11 patients for further analysis. Pre- and postdilution was equally distributed between the groups; patient characteristics and initial ventilator settings are shown in Tables 1 and 2. Most of our patients were ventilated in assist-control mode (9 out of 11), two were ventilated in pressure support mode. All patients were ventilated in the spirit of a lung protective ventilation strategy using low tidal volumes; mean tidal volume at baseline was 6.36 ± 0.67 ml/kg ideal bodyweight.
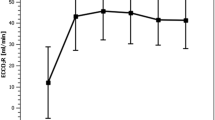
At baseline minute ventilation appeared to be 10.1 l/min (7.0–12.9 l/min), during CRRT-ECCO2R therapy a significant reduction in minute ventilation was observed to 9.2 l/min at 1 h (5.9–12.4 l/m, p < 0.05), at 3 h there was still a reduction compared to baseline to 8.4 l/min (5.1–14.2 l/min), which was not significant (p = 0.052), at 6 h there was still a reduction compared to baseline to 9.6 l/min (7.5–15.3 l/min), again not significant (p = 0.450) (Fig. 1).
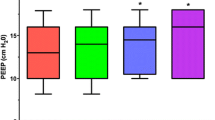
Composite figure of the analyzed parameters; each figure shows the evolution of the parameter displayed during the course of the trial relative compared to baseline. Parameters do not shape in any consistent manner but show a trend towards reductions after 3 h of therapy. This is conclusive to the statistical measures performed. Pmean mean airway pressure, Pinsp maximum inspiratory pressure, Vt tidal volume, paCO2 arterial partial pressure of carbon dioxide
Respiratory rate was 23.6 bpm (18–39 bpm) at baseline. It was reduced to 21.1 bpm (15–26 bpm) at 1 h, to 23 bpm (17–34 bpm) at 3 h and increased slightly to 24.8 bpm (18–36 bpm) at 6 h into therapy, none of these changes reached significance (Kendall p = 0.127).
Tidal volume was 425.5 ml (300–500 ml) at baseline. It increased to 440.6 ml (346–600 ml, p = 0.750) after 1 h and decreased significantly to 364.1 ml (258–450 ml, p < 0.05) after 3 h. After 6 h of therapy we found a decrease to 395 ml (305–550 ml) still, but this decrease was not statistically significant (p = 0.240).
There was a statistically significant decrease in paCO2 from 34.4 mmHg (29–49 mmHg) at baseline to 28.8 mmHg (20–40 mmHg, p < 0.05) at 1 h, a statistically meaningful decrease to 31.7 mmHg (27–37 mmHg, p < 0.05) was observed at 3 h, which kept constant to 31.6 mmHg (27–37 mmHg, p = 0.164) at 6 h during CRRT-ECCO2R (Fig. 2).
Combined whiskers-boxplots of studies parameters throughout the trial period. Differences in respiratory rates over time are marginal with no decrease found. Moreover, an increase is seen after 6 h. We do not have a conclusive explanation for this fact. In the first hour there was no difference in Pinsp, after 3 h the median decreased but the values also spread wider, strangely enough Pinsp increased after 6 h, we do not have an explanation for this. Counter intuitively the power applied did not decrease although CO2 was actually removed from the body, but it also did not increase. A clear decrease in arterial CO2 is seen after 1 h of therapy as expected due to extracorporeal CO2 removal. Nevertheless, CO2 is nearly back to baseline values at 3 h and stays at this level until 6 h of therapy. One explanation may be oxygenator exhaustion, but we did not have any hint towards this. paCO2 arterial partial pressure of carbon dioxide, Pinsp maximum inspiratory pressure
We also calculated delta pressure, which was also not significantly different between data-acquisition-points (Kendall p = 0.143, data not shown).
The power transmitted to the lungs owing to ventilator settings at each data-acquisition-point was calculated. We found the values to be 18.4 J/min (11.8–34 J/min) at baseline, 17.8 J/min (8.6–29.8 J/min, p = 0.695) after 1 h, 15 J/min (6.2–32.8 J/min, p = 0.054) after 3 h, and 16.3 J/min (8.0–23.5 J/min, p = 0.175) after 6 h of therapy.
Total CO2 removal of the tested device averaged over all recordings was 20.7 ml CO2/min (18.7–23.8 ml CO2/min) regardless of the mode of dilution applied.
Regarding the mode of dilution, we found no significant difference between predilution (CO2 elimination 19.4 ml CO2/min, 15.0–26.1 ml CO2/min) and postdilution (CO2 elimination 23.1 ml CO2/min, 17.5–28.7 ml CO2/min) (p = 0.26) (Tables 3, 4).
No complications or adverse events occurred during conduction of the trial.
Discussion
We used a combined approach that incorporates extracorporeal CO2 removal in a system for hemodialysis (CRRT-ECCO2R). In patients with combined renal and respiratory failure this system was in our trial capable to safely reduce minute ventilation statistically significant in the first hour of therapy. Nevertheless, it was not able to keep or even improve this result throughout the next hours of our trial. Furthermore, it is debatable if a mean reduction of 0.8 l/min although statistically significant is clinically meaningful. Especially because the statistically significant reduction in tidal volume appeared 2 h later at 3 h into therapy, when the decrease in minute ventilation was not statistically significant.
This argument gets even stronger when taking into account that CO2 decreased significantly also at that exact point in time. But there is no significant corresponding decrease in respiratory rate. Calculating the power transmitted, although the formula has not been widely used yet, shows the same result; there is a non-significant decrease in power at this exact time.
Terragni et al. using the same device [13] found significant reductions in minute ventilation and concluded that CRRT-ECCO2R is sufficient to facilitate lung-protective ventilation. In their trial, a fixed tidal volume of 6 ml/kg was aimed and reached. After achieving this goal, the team removed CO2 in the intervention group and found they were able to manage the increased CO2 load produced by decreased tidal volumes. Looking at their data it becomes obvious that only a fixed amount of CO2 was removed bringing CO2 back to baseline before tidal volumes were reduced. Our approach was different. We adopted the ventilator settings as they stood and removed CO2 first. Treating physicians were blinded to whether CO2 removal was active or not. They were not briefed about the trial but kept going with routine practice to ventilate a patient according to the ALARA principle (as-low-as-reasonably-achievable) we use in our unit. We think our approach is much less of a laboratory situation but reflects everyday life closer than the approach Terragni et al. used. The authors were able to show, that in a controlled environment, CRRT-ECCO2R enables treating physicians to decrease tidal volume and control for increased carbon dioxide via the extracorporeal circuit. In our uncontrolled situation CO2 was removed first and we observed, if lung protective ventilation resulted. In this setting, we were not able to show statistical significance, although absolute trends towards protective ventilation could be found. Data provided in the aforementioned article do not allow for calculation of applied power retrospectively.
We were able to confirm the findings of Terragni et al. in that both studies show a constant portion of CO2 being removed by this system. As expected by the minimal-flow, the amount of CO2 elimination is limited. The capacity to remove CO2 we found is likely to be insufficient in some cases. Since there is only very little experience with this system and technique, further evidence must be generated. Another drawback is the very limited amount of oxygenation at these flow rates making it feasible for patients with partial respiratory insufficiency only.
Furthermore, we evaluated the effect of pre- versus postdilution on CO2-removal. By theory, predilution would be more efficient in terms of CO2-removal than postdilution because higher efflux rates can be achieved. In spite of this theory we did not find any significant difference in CO2-removal between the two modes. One possible explanation could be a suboptimal membrane lung, so that pre- vs. postdilution should be tested using another membrane lung to verify or falsify this possibility.
There are limitations to our study. First, our sample size was small; we might have missed statistical significance for this reason, although this seems unlikely by looking at our data. Second, this was a feasibility study; we did not have a control group. Third, inclusion criteria were kept very broad so that we included only due to the need of CRRT and mechanical ventilation. All patients included were not in need for ECCO2R, they did find without it before and after. We, therefore, do not know if the system works for patients with a heavy CO2 load. On the other hand, this is strength as well, because we can largely exclude recruiting bias. From our results one can see that CO2 removal consistently was 21 ml CO2/min. This is only about one-tenth of the normal resting CO2 production in an adult. Taking this into account it is very unlikely that a significant amount of CO2 can be removed using this system in a severely hypercarbic patient who would need it the most.
After all, experience is limited with these jack-of-all-trades devices, making it hard to draw general conclusions on their effectiveness. Further research needs to be conducted with those systems to find the right patients benefiting from it, since from our results shown here one cannot conclude that hypercarbic patients will benefit from the small amount of CO2 removal we found.
Conclusion
Based on our current knowledge the least harmful approach to mechanical ventilation should be chosen to avoid ventilator-induced injury. Although extracorporeal CO2 removal using minimal-flow systems suffers from a limited efficiency, the concept of reduced invasiveness, which is enabled by vascular access with smaller cannulas, is appealing. This is especially true since fusing CRRT and ECCO2R in patients that require both treatments might enhance the benefit.
With our study we were able to find detached statistically significant reductions in minute ventilation, tidal volume, and paCO2. In our study, we found no significant decrease in applied power besides aforementioned statistically significant reductions in surrogates for lung protection during mechanical ventilation. From our trial, we need to draw the conclusion that ECCO2R using minimal flow rates with that merely small amount of CO2 removal we found is most likely not sufficient to benefit patients with heavy CO2 load.
References
Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8.
Hickling KG, Henderson SJ, Jackson R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med. 1990;16:372–7.
Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Resp Crit Care Med. 2007;175:160–6.
Gattinoni L, Marini JJ, Pesenti A, Quintel M, Mancebo J, Brochard L. The “baby lung” became an adult. Intensive Care Med. 2016;42:663–73.
Bein T, Weber-Carstens S, Goldmann A, Muller T, Staudinger T, Brederlau J, et al. Lower tidal volume strategy (approximately 3 ml/kg) combined with extracorporeal CO2 removal versus ‘conventional’ protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med. 2013;39:847–56.
Guldner A, Kiss T, Bluth T, Uhlig C, Braune A, Carvalho N, et al. Effects of ultraprotective ventilation, extracorporeal carbon dioxide removal, and spontaneous breathing on lung morphofunction and inflammation in experimental severe acute respiratory distress syndrome. Anesthesiology. 2015;122:631–46.
Gattinoni L, Tonetti T, Cressoni M, Cadringher P, Herrmann P, Moerer O, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med. 2016;42:1567–75.
Horie S, Ansari B, Masterson C, Devaney J, Scully M, O’Toole D, et al. Hypercapnic acidosis attenuates pulmonary epithelial stretch-induced injury via inhibition of the canonical NF-kappaB pathway. Intensive Care Med Exp. 2016;4:8.
Feihl F, Eckert P, Brimioulle S, Jacobs O, Schaller MD, Melot C, et al. Permissive hypercapnia impairs pulmonary gas exchange in the acute respiratory distress syndrome. Am J Resp Crit Care Med. 2000;162:209–15.
Crystal GJ. Carbon dioxide and the heart: physiology and clinical implications. Anesth Analg. 2015;121:610–23.
Moerer O, Quintel M. Protective and ultra-protective ventilation: using pumpless interventional lung assist (iLA). Min Anestesiol. 2011;77:537–44.
Terragni P, Ranieri VM, Brazzi L. Novel approaches to minimize ventilator-induced lung injury. Curr Opin Crit Care. 2015;21:20–5.
Terragni PP, Del Sorbo L, Mascia L, Urbino R, Martin EL, Birocco A, et al. Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology. 2009;111:826–35.
Hill JD, O’Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med. 1972;286:629–34.
Curley GF. The goldilocks principle, carbon dioxide, and acute respiratory distress syndrome: too much, too little, or just. Right? Anesthesiology. 2016;124:532–4.
Mielck F, Quintel M. Extracorporeal membrane oxygenation. Curr Opin Crit Care. 2005;11:87–93.
Vincent JL. Acute kidney injury, acute lung injury and septic shock: how does mortality compare? Contrib Nephrol. 2011;174:71–7.
Bagshaw SM. Epidemiology of renal recovery after acute renal failure. Curr Opin Crit Care. 2006;12:544–50.
Batchinsky AI, Jordan BS, Regn D, Necsoiu C, Federspiel WJ, Morris MJ, et al. Respiratory dialysis: reduction in dependence on mechanical ventilation by venovenous extracorporeal CO2 removal. Crit Care Med. 2011;39:1382–7.
Cardenas VJ Jr, Miller L, Lynch JE, Anderson MJ, Zwischenberger JB. Percutaneous venovenous CO2 removal with regional anticoagulation in an ovine model. ASAIO J (Am Soc Artif Intern Organs 1992). 2006;52:467–70.
Gattinoni L, Agostoni A, Pesenti A, Pelizzola A, Rossi GP, Langer M, et al. Treatment of acute respiratory failure with low-frequency positive-pressure ventilation and extracorporeal removal of CO2. Lancet. 1980;2:292–4.
Gille JP, Bauer P, Bollaert PE, Tousseul B, Kachani-Mansour R, Munsch L. CO2 removal with hemodialysis and control of plasma oncotic pressure. ASAIO Trans/Am Soc Artif Intern Organs. 1989;35:654–7.
Habashi NM, Borg UR, Reynolds HN. Low blood flow extracorporeal carbon dioxide removal (ECCO2R): a review of the concept and a case report. Intensive Care Med. 1995;21:594–7.
Isobe J, Mizuno H, Matsunobe S, Shimizu Y, Ikada Y, Kishida A. A new type of low blood flow ECCO2R using a hemodialysis system in apneic states. ASAIO Trans/Am Soc Artif Intern Organs. 1989;35:638–9.
Mancini P, Whittlesey GC, Song JY, Salley SO, Klein MD. CO2 removal for ventilatory support: a comparison of dialysis with and without carbonic anhydrase to a hollow fiber lung. ASAIO Trans/Am Soc Artif Intern Organs. 1990;36:M675-8.
Matsunobe S, Isobe J, Mizuno H, Shimizu Y. Extracorporeal CO2 removal by hemodialysis in patients with chronic respiratory failure. ASAIO Trans/Am Soc Artif Intern Organs. 1987;33:441–5.
Morris JL, Rosen DA, Calvert KS, Gustafson RA, Steelman RJ, Rosen KR, et al. Extracorporeal CO2 removal in a child with a single ventricle by the addition of an oxygenator to a dialysis circuit. Pediatr Crit Care Med. 2003;4:104–6.
Quintard JM, Barbot O, Thevenot F, de Matteis O, Benayoun L, Leibinger F. Partial extracorporeal carbon dioxide removal using a standard continuous renal replacement therapy device: a preliminary study. ASAIO J (Am Soc Artif Intern Organs 1992). 2014;60:564–9.
Svitek RG, Federspiel WJ. A mathematical model to predict CO2 removal in hollow fiber membrane oxygenators. Ann Biomed Eng. 2008;36:992–1003.
Zanella A, Patroniti N, Isgro S, Albertini M, Costanzi M, Pirrone F, et al. Blood acidification enhances carbon dioxide removal of membrane lung: an experimental study. Intensive Care Med. 2009;35:1484–7.
Zanella A, Mangili P, Giani M, Redaelli S, Scaravilli V, Castagna L, et al. Extracorporeal carbon dioxide removal through ventilation of acidified dialysate: an experimental study. J Heart Lung Transpl. 2014;33:536–41.
Zanella A, Castagna L, Salerno D, Scaravilli V, Abd El Aziz El Sayed Deab S, Magni F, et al. Respiratory electrodialysis. a novel, highly efficient extracorporeal CO2 removal technique. Am J Resp Crit Care Med. 2015;192:719–26.
Douglas AR, Jones NL, Reed JW. Calculation of whole blood CO2 content. J Appl Physiol. 1988;65:473–7.
Author information
Authors and Affiliations
Contributions
OM initiated, designed and helped conducting the study, was involved in statistical analysis and interpreting the data, wrote and revised the manuscript. LOH condensed and analyzed the data and drafted and revised the manuscript. JB performed data acquisition and analysis and revised the manuscript. DH helped interpreting and analyzing the data. JH helped acquiring and analyzing data and revised the manuscript. MQ initiated and designed the study and was involved in data analysis and revising the manuscript. All authors agree to be accountable for all aspects of the work and have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest and funding information
The study was fully financed by departmental funding; the EQUAsmart® system was kindly provided by hemodec, Italy. The company was not further involved in conduction of the trial, data acquisition, statistical analysis or interpretation of the data. The Department of Anaesthesiology, University of Göttingen provided educational courses on lung protective ventilation and hemodynamic monitoring partially supported by Maquet Critical Care, CareFusion, and Pulsion Medical. Prof. Quintel consults for companies related to the spectrum of Critical Care Medicine including manufacturers (Maquet Critical Care, CareFusion, Pulsion Medical Systems, Novalung, CytoSorbents, Gambro) and is compensated for these consultations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moerer, O., Harnisch, LO., Barwing, J. et al. Minimal-flow ECCO2R in patients needing CRRT does not facilitate lung-protective ventilation. J Artif Organs 22, 68–76 (2019). https://doi.org/10.1007/s10047-018-1068-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-018-1068-8