Abstract
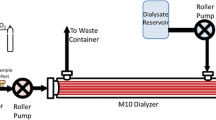
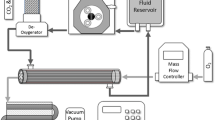
A mathematical model has been developed to predict CO2 removal in hollow fiber membrane oxygenators. The model is analogous to one developed previously for predicting O2 transfer. A mass transfer correlation was determined in water for O2 and CO2 exchange and collapsed onto one universal curve. The correlation was used to predict CO2 removal in blood by incorporating a ‘facilitated diffusivity’ to account for the transport of CO2 present as bicarbonate. The diffusion of bicarbonate greatly increased the ability of the oxygenator to remove CO2 in blood compared to water. A fiber bundle module was fabricated to test the model predictions. The fiber bundle had a length of 13 cm and a bundle thickness of 0.2 cm. The module was tested in bovine blood at flowrates of 0.75, 1.5, and 2.2 L/min and CO2 removal rate predictions were within 9% of experimental measurements at all flowrates. The O2 transfer rate predictions were within 10% of experimental measurements. A second module was manufactured with a bundle of length 4 cm and thickness of 1 cm. The CO2 removal predictions were within the standard deviation of the experimental measurements.








Similar content being viewed by others
References
Boschetti, F., et al., Blood flow pulsatility effects upon oxygen transfer in artificial lungs. Asaio J. 2003, 49(6): 678–686
Brunston, R. L., Jr., et al. Total arteriovenous CO2 removal: simplifying extracorporeal support for respiratory failure. Ann. Thorac. Surg. 64(6):1599–1604, 1997; discussion 1604-5.
Utley, J. R., et al. Cardiovascular implants and artificial organs—Blood-gas exchangers oxygenators. In: AAMI Standards and Recommended Practices: Biomedical Equipment, Part 1: General Safety and Design; Equipment for Therapy and Surgery. American National Standards Institute, Inc., 1996, pp. 633–648.
Dierickx, P. W., D. De Wachter, and P. R. Verdonck, Blood flow around hollow fibers. Int. J. Artif. Organs 2000, 23(9): 610–617
Federspiel, W. J., T. J. Hewitt, and B. G. Hattler, Experimental evaluation of a model for oxygen exchange in a pulsating intravascular artificial lung. Ann. Biomed. Eng. 2000. 28(2): 160–167
Federspiel, W., et al., Temporary support of the lungs: the artificial lung, in The Transplantation and Replacement of Thoracic Organs, D.K.C. Cooper, L.W. Miller, and G.A. Patterson, Editors. 1996, Kluwer Academic Publishers: Boston. pp 717–728
Forster, R. E., Diffusion of gases in the lungs. Physiologist 1966, 9(2): 110–122
Forster, R. E. and E. D. Crandall, Time course of exchanges between red cells and extracellular fluid during CO2 uptake. J. Appl. Physiol. 1975, 38(4): 710–718
Gage, K. L., et al., Predicting membrane oxygenator pressure drop using computational fluid dynamics. Artif. Organs 2002, 26(7): 600–607
Gartner, M. J., et al., Modeling flow effects on thrombotic deposition in a membrane oxygenator. Artif. Organs, 2000. 24(1): 29–36
Golob, J. F., et al., Acute in vivo testing of an intravascular respiratory support catheter. Asaio J. 2001, 47(5): 432–437
Guyton, A. C. Basic Human Physiology: Normal Function and Mechanisms of Disease, 2nd edn. Philadelphia: Saunders, xi, 931 pp, 1977.
Hattler, B. G., et al., A respiratory gas exchange catheter: in vitro and in vivo tests in large animals. J. Thorac. Cardiovasc. Surg. 2002, 124(3): 520–530
Hewitt, T. J., B. G. Hattler, and W. J. Federspiel, A mathematical model of gas exchange in an intravenous membrane oxygenator. Ann. Biomed. Eng. 1998, 26(1): 166–178
Kaar, J. L., et al., Towards improved artificial lungs through biocatalysis. Biomaterials 2007, 28(20): 3131–3139
Kays, W. M. and M. E. Crawford. Convective Heat and Mass Transfer, 3rd edn. McGraw-Hill series in mechanical engineering. New York: McGraw-Hill, xxxii, 601 pp, 1993.
Loeppky, J. A., U. C. Luft, and E. R. Fletcher, Quantitative description of whole blood CO2 dissociation curve and Haldane effect. Respir. Physiol. 1983, 51(2): 167–181
Mancini, P., 2nd, et al. CO2 removal for ventilatory support: a comparison of dialysis with and without carbonic anhydrase to a hollow fiber lung. ASAIO Trans. 36(3):M675–M678, 1990.
MatLab, Version 6.2. The MathWorks, 2003.
Mockros, L. F. and R. Leonard, Compact cross-flow tubular oxygenators. Trans. Am. Soc. Artif. Intern. Organs 1985, 31: 628–633
Salley, S. O., et al., Immobilized carbonic anhydrase in a membrane lung for enhanced CO2 removal. ASAIO Trans. 1990, 36(3): M486–M490
Spaeth, E. E., Blood oxygenation in extracorporeal devices: theoretical considerations. CRC Crit. Rev. Bioeng. 1973, 1(4): 383–417
Svitek, R. G., B. J. Frankowski, and W. J. Federspiel, Evaluation of a pumping assist lung that uses a rotating fiber bundle. Asaio J. 2005, 51(6): 773–780
Vaslef, S. and R. Anderson. The Artificial Lung, 1 edn. Gas Exchange in the Venous System: Support for the Failing Lung. Georgetown: Landes Bioscience, 2002.
Vaslef, S. N., et al., Use of a mathematical model to predict oxygen transfer rates in hollow fiber membrane oxygenators. Asaio J. 1994, 40(4): 990–996
Vaslef, S. N., et al., Computer-assisted design of an implantable, intrathoracic artificial lung. Artif. Organs 1994, 18(11): 813–817
Vaslef, S. N., et al., Design and evaluation of a new, low pressure loss, implantable artificial lung. Asaio J. 1994. 40(3): M522–M526
Wickramasinghe, S. R., M. J. Semmens, and E. L. Cussler, Mass transfer in various hollow fiber geometries. J. Memb. Sci. 1992, 69(3): 235
Wickramasinghe, S. R., et al., Designing blood oxygenators. Ann. N. Y. Acad. Sci. 2003, 984: 502–514
Zwischenberger, J. B., et al., Percutaneous extracorporeal arteriovenous CO2 removal for severe respiratory failure. Ann. Thorac. Surg. 1999, 68(1): 181–187
Zwischenberger, J. B., et al., Percutaneous extracorporeal arteriovenous carbon dioxide removal improves survival in respiratory distress syndrome: a prospective randomized outcomes study in adult sheep. J. Thorac. Cardiovasc. Surg. 2001, 121(3):542–551
Acknowledgments
This work was funded by the Commonwealth of Pennsylvania, the National Tissue Engineering Consortium (NTEC), U.S. Army Medical Research and Material Command Grant Number DAMD17-02-0717, and also by National Institutes of Health (NIH), the National Heart, Lung, and Blood Institute (NHLBI) Grant Number RO1 HL70051.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
CO2 Transport Equations
The following is a derivation of the convection–diffusion equations for CO2 in a blood continuum. The flowrate of dissolved CO2 and bound CO2 (bicarbonate and CO2 bound to hemoglobin) due to convection through a differential element, dA can be expressed as:
The flowrate of dissolved (free) CO2 and CO2 stored as HCO3 due to diffusion through dA is:
CO2 bound to hemoglobin will have low diffusion capacity due to the large size of the hemoglobin molecule. The total flowrate of CO2 through a differential volume element dV as dV → 0 is:
The dissolved component of CO2 will obey Henry’s law and can be expressed in terms of partial pressure and solubility\((\alpha_{{\rm CO}_2})\):
Using the chain rule and rearranging gives the transport equation for CO2 in blood:
The right hand side of Eq. (A.5) is the diffusion of dissolved CO2 and HCO3 and the left hand side is the convection of CO2 in all forms. The term in parenthesis on the right hand side is a facilitated diffusion coefficient:
The convective-diffusion equation for CO2 can be obtained by dividing the right hand side of Eq. (A.5) by \(1+(1/\alpha)(\partial C_{{\rm CO}_2}^b/\partial P_{{\rm CO}_2})\) assuming a constant value for \(\partial C_{{\rm CO}_2}^b/\partial P_{{\rm CO}_2}\) (approximated as \(\overline {\lambda _{{\rm CO}_2} } \) in the text):
The convective-diffusion equation leads to the definition of the effective diffusivity used in the Sc number:
Boundary Conditions
The solution to the convective diffusion equation requires a boundary condition at the surface of the fibers. The flux across the fiber wall is due to the diffusion of dissolved CO2 and bicarbonate ions. As the dissolved CO2 is removed by the fibers, the bicarbonate is rapidly converted to CO2 through the action of the enzyme carbonic anhydrase. At the fiber surface, the diffusional flux is:
which results in the definition of the Sh number.
Rights and permissions
About this article
Cite this article
Svitek, R.G., Federspiel, W.J. A Mathematical Model to Predict CO2 Removal in Hollow Fiber Membrane Oxygenators. Ann Biomed Eng 36, 992–1003 (2008). https://doi.org/10.1007/s10439-008-9482-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-008-9482-3