Abstract
A 6-year-old boy had cold-like symptoms and was diagnosed with influenza A at a clinic. Administration of oseltamivir and azithromycin did not improve the symptoms. He was referred to our hospital and was diagnosed with H1N1 pneumonia. The patient required ventilator support. However, hypoxia and hypercapnia were uncontrollable. To oxygenate and reduce the carbon dioxide concentration, veno-venous extracorporeal membrane oxygenation (ECMO) was applied 24 h after admission. We established outflow via the right internal jugular vein and inflow via the right femoral vein. Six hours later, an electrical storm of ventricular fibrillation occurred, probably due to influenza myocarditis. Chest compression was started immediately. Both cardioversion and medication were ineffective in treating the electrical storm. Therefore, we decided to switch the veno-venous ECMO to veno-arterial ECMO to maintain systemic flow. During chest compression, a 6-mm graft was anastomosed to the left common femoral artery, and an outflow tube was connected to the graft. Consequently, veno-arterial ECMO was established via outflow through the left common femoral artery and inflow through both the right jugular vein and right femoral vein. Veno-arterial ECMO terminated the electrical storm, and cardiac output improved. Veno-arterial ECMO was provided for 107 h, and was then replaced by veno-venous ECMO. Forty-three hours later, veno-venous ECMO was discontinued. The patient was successfully weaned from the mechanical ventilator on the 9th day after admission. Unfortunately, spinal infarction appeared as a complication. The patient was discharged from the hospital on the 86th day, and has now returned to primary school.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The novel swine-origin influenza A (H1N1) virus infection of 2009 rapidly led to a worldwide pandemic [1]. Myocarditis is a rare but life-threatening complication of influenza. Extracorporeal membrane oxygenation (ECMO) is sometimes used for treatment of myocarditis in adults. However, for children, it is difficult to apply ECMO because the vessel size of children is so small that cannulation for ECMO is difficult to achieve. Hence, median sternotomy is often performed to cannulate the ascending aorta and right atrium [2]. Here, we report the case of a child with influenza A-associated fulminant myocarditis who was rescued by ECMO through the peripheral vessels.
Case report
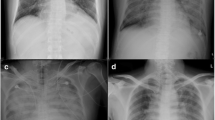
A 6-year-old boy who had fever and cough consulted a pediatric physician on December 4, 2009. He was diagnosed with influenza A. Administration of oseltamivir and azithromycin did not improve the symptoms and the patient was referred to our hospital on the next day. His body weight was 22 kg, height 125 cm, conscious level clear, body temperature 37.8°C, blood pressure 110/50 mmHg, heart rate 140/min, and respiratory rate 34/min. Hypoxia (PaO2/FiO2 111.9) and hypercapnia (PaCO2 113 mmHg) required mechanical ventilation. Hematologically, the values of white blood cell count, C-reactive protein, creatinine kinase, and troponin I were 15,400/μL, 3.7 mg/dL, 622 IU/L, and 4.14 ng/mL, respectively. On the plain chest X-ray, a ground-glass pattern was seen at the right upper lobe (Fig. 1). The electrocardiogram (ECG) showed ST elevation in V1-3. Ultrasonic echocardiography (UCG) demonstrated diffuse mild hypokinesia with a left ventricular ejection fraction (LVEF) of 50%. Consequently, ECMO was scheduled to oxygenate the patient and reduce the carbon dioxide concentration. In this case, veno-venous (V-V) ECMO was adopted because the left ventricular (LV) dysfunction was not thought to be severe. To apply V-V ECMO, a 14 Fr outflow cannula (Medtronic, Minneapolis, MN, USA) was inserted through the right jugular vein to the right atrium and a 16 Fr inflow cannula (Medtronic) was inserted through the right femoral vein. A centrifugal pump (Rotaflow, Maquet) and an oxygenator (Biocube2000, Nipro) were applied. All devices were heparin-coated. The total priming volume including pump, oxygenator, and tube was 212 mL; a pump flow of 3,200 rpm generated an output of 1.8 L/min. Intravenous heparin was given, aiming at an activated clotting time of 150–200 s. The data of the arterial blood gas showed pH 7.262, PO2 258.2 mmHg, PCO2 52.3 mmHg, HCO3 23.8 mmol/L, base excess −2.8 mmol/L, and lactate 16 mg/dL a half hour after the commencement of V-V ECMO. The FiO2 of V-V ECMO and respirator were 1.0 and 0.7, respectively. Six hours after commencing the V-V ECMO, an electrical storm of ventricular fibrillation occurred, probably due to influenza myocarditis. Chest compression was immediately started. Both cardioversion and medical treatments had no effect. Consequently, we decided to switch the V-V ECMO to veno-arterial (V-A) ECMO to maintain systemic flow. During chest compression, a 6-mm polytetrafluoroethylene graft (W.L. Gore and Associates, Flagstaff, AZ, USA) was anastomosed to the left common femoral artery (CFA) to be used as outflow. This anastomosis was difficult because this procedure was performed during chest compression and the size of the left CFA was 5 mm. Both cannulae were used for the inflow through the right internal carotid vein and right common femoral vein. The total chest compression time was 141 min. After 107 h of support by V-A ECMO, the UCG showed an improved LVEF (50%), which allowed alteration to V-V ECMO. Another 43 h later, V-V ECMO was discontinued. Pulse therapy of methyl prednisolone (30 mg kg−1 day−1) and administration of γ-globulin (500 mg kg−1 day−1) were performed during the ECMO period. The patient’s hemodynamic state remained stable after the discontinuation of ECMO. The next day, the patient was successfully weaned from the respirator. However, spinal infarction at the level of Th4-5 occurred as a complication (Fig. 2). Spinal infarction caused paraplegia and disturbance of the bladder and rectum. After difficult rehabilitation, he was discharged from the hospital on the 86th day after administration with a LVEF of 79%. He is currently re-enrolled in primary school.
Discussion
Myocarditis associated with influenza A is a rare but life-threatening disease. It often leads to poor cardiac function and lethal arrhythmia. A number of reports have shown the effectiveness of mechanical circulatory support systems, for example intra-aortic balloon pumps, ECMO, and ventricular assist devices [3, 4]. These mechanical support systems are used to maintain cardiac output and organ perfusion and to minimize the need for inotropic support until myocardial recovery. However, they are sometimes unavailable for small children because the size of their vessels is too small. The results of the national survey of fulminant myocarditis in Japan showed that 57.7% of patients with fulminant myocarditis treated with V-A ECMO survived and returned to regular life [5]. The optimum timing of ECMO application has been controversial, but it is important to clarify this because rapid hemodynamic deterioration could occur. Freedman et al. [6] reported that 57% of patients with myocarditis were originally diagnosed with pneumonia or asthma, and the sensitivities of ECG and chest radiograph were 93 and 55%, respectively. In our case, V-V ECMO was chosen as a mechanical support against uncontrollable hypoxia and hypercapnia. During application of ECMO, the LVEF was 50% and LV wall motion demonstrated diffuse mild hypokinesia on the UCG. Because LV dysfunction was not severe, we were unsure whether V-V ECMO or V-A ECMO should be applied. Moreover, distal leg ischemia is a significant complication after femoral artery cannulation. Ko et al. [7] reported that limb ischemia was noted in 26% of adult cardiac patients on ECMO support. In particular, limb ischemia can easily occur in small children. Accordingly, cannulations through the ascending aorta and right atrium via median sternotomy are often used for such small children. However, the disadvantages of median sternotomy are infection and hemorrhage. Our team, which included cardiovascular surgeons, pediatric cardiologists, and medical engineers, has tried to perform ECMO through peripheral vessels even for small children to avoid median sternotomy; thus, small size cannulae were conventionally prepared. In this case, after a 6-h support via V-V ECMO, it was switched to V-A ECMO because an electrical storm occurred, probably due to myocarditis. Although LV dysfunction is the most common abnormality of myocarditis, the diagnosis of myocarditis is sometimes difficult to make in the early stage of the disease. In general, V-A ECMO is used when cardiac support is necessary. In this case, ECMO was introduced to treat hypoxia and hypercapnia. The results of the examinations demonstrated the high value of troponin I, but the LVEF was 50%. We hesitated to apply V-A ECMO because the size of the carotid and femoral arteries was small and the patient’s cardiac function had not severely deteriorated. In retrospect, his cardiac function at the time of initiation of ECMO might have been overestimated. Moreover, spinal infarction occurred as a complication. This might be related to the hypotension during the chest compression when the electrical storm occurred. After chest compression for 141 min, V-A ECMO was established. If V-A ECMO had been chosen from the beginning, spinal infarction might have been avoided. Therefore, V-A ECMO should probably have been applied as a mechanical support from the beginning.
Conclusion
We encountered a pediatric patient with influenza myocarditis who was rescued by utilization of ECMO. Immediate application of circulatory support is the key to saving patients with myocarditis.
References
Novel swine-origin Influenza A (H1N1) virus investigation team. Novel swine-origin influenza A (H1N1) virus investigation team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Eng J Med. 2009;360:2605–15.
Tokunaga S, Morita S, Masuda M, Tomita Y, Nishida T, Tominaga R. How to cope with the pitfalls of extracorporeal membrane oxygenation support: case of a girl with fulminant myocarditis. J Artif Organs. 2007;10:115–7.
Taoka M, Shiono M, Hata M, Sezai A, Iida M, Yoshitake I, Wakui S, Negishi N, Sezai Y, Minami K. Child with fulminant myocarditis survived by ECMO support. Ann Thorac Cardiovasc Surg. 2007;13:60–4.
Topkara VK, Dang NC, Barili F, Martens TP, George I, Cheema FH, Bardakci H, Ozcan AV, Naka Y. Ventricular assist device use for the treatment of acute viral myocarditis. J Thorac Cardiovasc Surg. 2006;131:1190–1.
Aoyama N, Izumi T, Hiramori K, Isobe M, Kawana M, Hiroe M, Hishida H, Kitaura Y, Imaizumi T. National survey of fulminant myocarditis in Japan: therapeutic guidelines and long-term prognosis of using percutaneous cardiopulmonary support for fulminant myocarditis. Circ J. 2002;66:133–44.
Freedman SB, Haladyn JK, Floh A, Kirsh JA, Taylor G, Thull-Freedman J. Pediatric myocarditis: emergency department clinical findings and diagnostic evaluation. J Pediatrics. 2007;120:1278–85.
Ko WJ, Lin CY, Chen RJ, Wang SS, Lin FY, Chen YS. Extracorporeal membrane oxygenation support for adult postcardiotomy cardiogenic shock. Ann Thorac Surg. 2002;73:538–45.
Acknowledgments
We are deeply grateful to Andrew Hamilton for his cooperation in editing.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Oda, T., Yasunaga, H., Tsutsumi, Y. et al. A child with influenza A (H1N1)-associated myocarditis rescued by extracorporeal membrane oxygenation. J Artif Organs 13, 232–234 (2010). https://doi.org/10.1007/s10047-010-0523-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-010-0523-y