Abstract
An approach, combining several geochemical methods, was used to determine the groundwater properties and components of a hypogene karst system, where sampling is restricted only to the spring sites, and with a limited number of available sampling locations. Radiogenic isotopes (3H, 14C) were used to constrain the groundwater mean residence time and separate different groundwater components. Noble gases, stable isotopes of water (δ18O, δ2H), dissolved inorganic carbon (δ13C) and dissolved sulfate (δ34S, δ18O), and major ion and trace element composition were used to identify the source of water, its chemical evolution and water–rock interactions, as well as to identify the contribution and composition of endogenic gases. This approach was applied to three low-temperature thermal springs located in Mariovo (North Macedonia) associated with fossil hypogene caves, previously identified by morphological and geochemical studies of caves and cave deposits. Based on the obtained results, the main studied springs represent an output part of a regional hypogene karst groundwater system with a deep-circulating (~1 km), old (~15 ka), thermal (≥60 °C) water, which mixes with young (<50 years), cold (<14 °C) and shallow epigene karst groundwater. The output parts are structurally controlled, at the interception of low topography and deep faults, along which the groundwater interacts with deep-seated gases, dominantly CO2 of metamorphic origin (δ13C of +4.5‰ VPDB), with some contribution of mantle helium. The thermal karst groundwater interacts at depth with volcanic rocks from the nearby Neogene-Quaternary volcanic complex, as well as with metamorphic basement rocks and granitoids.
Résumé
Une approche combinant plusieurs méthodes géochimiques a été utilisée pour déterminer les propriétés et les composants des eaux souterraines d’un système karstique hypogène, dans lequel l’échantillonnage est réduit uniquement aux sites des sources, et avec un nombre limité d’emplacements disponibles pour l’échantillonnage. Les isotopes radiogéniques (3H, 14C) ont été utilisés pour contraindre le temps de résidence moyen des eaux souterraines et séparer les différents composants des eaux souterraines. Les gaz nobles, les isotopes stables de l’eau (δ18O, δ2H), le carbone inorganique dissous (δ13C), le sulfate dissous (δ34S, δ18O) et la composition en ions majeurs et éléments traces ont été utilisés pour identifier l’origine de l’eau, son évolution chimique et les interactions eau-roche, ainsi que la contribution et la composition des gaz endogènes. Cette approche a été appliquée à trois sources thermales basse température situées à Mariovo (Nord Macédoine) en relation avec des cavités hypogènes fossiles, précédemment identifiées par des études morphologiques et géochimiques des cavités et des dépôts en cavité. D’après les résultats obtenus, les principales sources étudiées représentent une partie des sorties du système souterrain karstique hypogène régional, avec une circulation d’eau profonde (~1 km), ancienne (~15,000 ans), thermale (≥60 °C), qui se mélange avec une eau souterraine jeune (<50 ans), froide (<14 °C) et peu profonde d’un karst épigène. Les sorties sont structuralement contrôlées à l’intersection des points bas de la topographie et des failles profondes le long desquelles les eaux souterraines interagissent avec les gaz gisant en profondeur, principalement le CO2 d’origine métamorphique (δ13C of +4.5‰) avec un certain apport de l’hélium du manteau. Les eaux souterraines du karst thermal interagissent en profondeur avec les roches appartenant au complexe volcanique du Néogène-Quaternaire voisin, ainsi qu’avec des roches du socle métamorphique et des granitoïdes.
Resumen
Se utilizó un método de combinación de varios métodos geoquímicos para determinar las propiedades y los componentes de las aguas subterráneas de un sistema kárstico hipogénico, en el que el muestreo se limita únicamente a los manantiales y con un número limitado de sitios de toma de muestras. Se utilizaron isótopos radiogénicos (3H, 14C) para acotar el tiempo medio de residencia de las aguas subterráneas y separar sus diferentes componentes. Se utilizaron gases nobles, isótopos estables del agua (δ18O, δ2H), carbono inorgánico disuelto (δ13C) y sulfato disuelto (δ34S, δ18O) y la composición de iones y oligoelementos principales para identificar la fuente de agua, su evolución química y las interacciones agua-roca, así como para identificar la contribución y la composición de los gases endógenos. Este enfoque se aplicó a tres manantiales termales de baja temperatura situados en Mariovo (Macedonia septentrional) asociados a cuevas hipogénicas fósiles, previamente identificadas mediante estudios morfológicos y geoquímicos y depósitos en cuevas. Sobre la base de los resultados obtenidos, los principales manantiales estudiados representan una parte de la salida de un sistema regional de aguas subterráneas hipogénicas kársticas con un agua subterránea de circulación profunda (~1 km), antigua (~15 ka), termal (≥60 °C), que se mezcla con aguas subterráneas kársticas jóvenes (<50 años), frías (<14 °C) y poco profundas del epígeno-kárstico. Las zonas de descarga están estructuralmente controladas, en la interceptación de la topografía baja y las fallas profundas, a lo largo de las cuales las aguas subterráneas interactúan con gases de gran profundidad, predominantemente CO2 de origen metamórfico (δ13C de +4.5‰ VPDB), con algún aporte de helio del manto. Las aguas subterráneas termales del karst interactúan en profundidad con las rocas volcánicas del complejo volcánico neógeno cuaternario, así como con las rocas metamórficas del subsuelo y los granitoides.
摘要
将多种地球化学方法综合考虑,以此确定深层岩溶系统的地下水属性和组分,该岩溶系统的采样仅限于泉水站点,并且可用于采样点的数量有限。用放射性同位素(3H,14C)来约束地下水的平均停留时间并分离出不同的地下水组分。稀有气体,稳定的水同位素(δ18O,δ2H),溶解的无机碳(δ13C)和溶解的硫酸盐(δ34S,δ18O)以及主要的离子和微量元素组成可用于识别水的来源,化学演化和水-岩石相互作用,以及识别内生气体的贡献和组成。该方法应用于位于Mariovo(北马其顿)的三个低温温泉,这些温泉与老的深层洞穴有关,先前已通过对洞穴和洞穴沉积物的形态和地球化学研究确定了这些温泉。根据获得的结果,研究的主要泉水代表区域性深层岩溶地下水系统的输出部分,其中深循环水(~1 km),古水(~15 ka),热水(≥60 °C),其中热水混合了新水(<50年),冷水(<14° C)和浅的表生岩溶地下水。输出部分在低地形和深断层的交汇处受到结构控制,沿其地下水与深层气体相互作用,深层气体主要是变质成因的二氧化碳(δ13C为+4.5‰ VPDB),并贡献了地幔氦。热岩溶地下水与附近新近纪-第四纪火山混合体的火山岩以及变质基底岩和花岗岩类在深部相互作用。
Resumo
Uma abordagem, combinando vários métodos geoquímicos, foi usada para determinar as propriedades da água subterrânea e os componentes de um sistema cárstico hipogênico, onde a amostragem é restrita apenas aos locais da nascente, e com um número limitado de locais de amostragem disponíveis. Isótopos radiogênicos (3H, 14C) foram usados para restringir o tempo médio de residência da água subterrânea e separar diferentes componentes da água subterrânea. Gases nobres, isótopos estáveis de água (δ18O, δ2H), carbono inorgânico dissolvido (δ13C) e sulfato dissolvido (δ34S, δ18O) e composição de íons principais e oligoelementos foram usados para identificar a fonte de água, sua evolução química e interações rochosas, bem como identificar a contribuição e composição dos gases endogênicos. Esta abordagem foi aplicada a três fontes termais de baixa temperatura localizadas em Mariovo (Norte da Macedonia) associadas a cavernas hipogênicas fósseis, previamente identificadas por estudos morfológicos e geoquímicos de cavernas e depósitos em cavernas. Com base nos resultados obtidos, as principais nascentes estudadas representam uma parte de saída de um sistema regional de águas subterrâneas hipogênicas cársticas com uma circulação profunda (~1 km), antiga (~15 ka), água termal (≥60 °C), que se mistura com água subterrânea cárstica jovem (<50 anos), fria (<14 °C) e epígena rasa. As partes de saída são estruturalmente controladas, na interceptação de baixa topografia e falhas profundas, ao longo das quais as águas subterrâneas interagem com gases profundos, predominantemente CO2 de origem metamórfica (δ13C de + 4.5‰ VPDB), com alguma contribuição de hélio do manto. A água subterrânea cárstica térmica interage em profundidade com as rochas vulcânicas do complexo vulcânico Neogeno-Quaternário próximo, bem como com rochas metamórficas do embasamento e granitóides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hypogene karstification is a relatively recent scientific paradigm in the understanding of the formation of karst systems (e.g. Klimchouk et al. 2017 and references therein). It addresses karst systems that develop due to bedrock dissolution by groundwater that recharges the karst rock formation from below (Klimchouk 2007). In contrast to the much more widespread epigene karst, where the chemical capacity to dissolve the bedrock is dominantly due to dissolved CO2 acquired at the surface, more diverse genetic mechanisms are present in hypogene karst systems due to a variety of geochemical processes (Ford and Williams 2007; Klimchouk 2007; Palmer 2007).
As hypogene karst systems generally become accessible only after being intercepted by surface erosional processes, the most common approach to studying them is to characterize the cave morphology and deposits in fossil cave systems (e.g. Audra et al. 2002; De Waele et al. 2016; Plan et al. 2012; Spötl et al. 2016; Temovski et al. 2013). A complementary approach is to study the geochemical properties of the groundwater associated with hypogene karst systems.
Various geochemical methods and approaches have been used in the study of groundwater systems (Clark 2015). Many of them have been also applied to groundwater in carbonate aquifers that might be associated with hypogene karst processes (i.e. aquifers with thermal waters, high gas concentrations etc.), in order to identify sources of fluids, flow-paths and geochemical processes. A combined use of hydrochemistry and stable isotopes, including sulfur and strontium isotopes, has been applied to identify water–rock interactions and separate different groundwater flows associated with the thermal springs in Derbyshire, England, UK (Gunn et al. 2006). Gary and Sharp (2006) identified interaction of the groundwater at Sistema Zacatón (Mexico) with Pleistocene volcanic rocks based on strontium isotopes, and related the speleogenesis of the deep phreatic caves to volcanic derived H2S and CO2. Hydrochemistry and isotopic composition of dissolved inorganic carbon has been used to identify the source and amount of geogenic CO2 related to the carbonate aquifers in the Appenine Region in Italy (e.g. Chiodini et al. 2000). A similar approach, combined also with noble gases, was used to identify geogenic gases in groundwater related to the giant collapse dolines in the Konya Closed Basin, Turkey (Bayari et al. 2009b, 2017), and sources of fluids in the deep phreatic cave Hranicka Abyss in Czechia (Sracek et al. 2019). Wynn et al. (2010) used sulfur isotopes to identify the sources of dissolved sulfur species in the thermal waters associated with sulfuric acid speleogenesis at Cerna Valley, Romania. Different radionuclides have been used to identify groundwater flow-paths and their mean residence times. Erőss et al. (2012) applied uranium, radium and radon to identify mixing of fluids in the Buda thermal karst system (Hungary) and to estimate the temperature and chemical composition of the end-members. Radiocarbon and tritium have been used to estimate the mean residence time of groundwater such as in the Bükk thermal karst system, Hungary (Hertelendi et al. 1995), the coastal thermal springs of Apulia, Italy (Santaloia et al. 2016), and the Konya Closed Basin in Tukey (Bayari et al. 2009a).
The approach in many of these examples involves using a relatively high number of sampling locations and/or time series of certain geochemical parameters covering longer periods (e.g. Bayari et al. 2009a, b; Erőss et al. 2020; Galdenzi et al. 2008; Mádl-Szőnyi and Tóth 2015). Spring waters often provide the main insight into the geochemical properties of these hypogene karst groundwater systems (e.g. Gunn et al. 2006; Mádl-Szőnyi and Tóth 2015; Palmer et al. 2017; Santaloia et al. 2016), due to their ease of access or due to lack of available boreholes. However, these discharge areas, generally located at points of low topography and controlled by major structural elements (Klimchouk 2007), are zones of convergence of discharge from different groundwater flow systems (local, intermediate and regional), allowing for mixing of groundwater with different geochemical composition (e.g. Erőss et al. 2012; Minissale et al. 2002). In such cases, to study the geochemical properties of a distinctive groundwater flow system, information about the geochemical composition of the end-members and their contributing ratios is required, which is not always attainable.
Here, an approach is presented of a combined use of several geochemical methods in the study of groundwater associated with hypogene karst systems, where sampling of the groundwater systems is restricted to only a small number of springs. Such an approach allowed for identification of the different components of groundwater where the end-member geochemical compositions were unknown. The approach was applied to Mariovo area in North Macedonia, where hypogene speleogenesis has been previously demonstrated based on morphological and geochemical studies of caves and cave deposits, and dissolution of carbonate rock due to cooling of CO2-rich thermal groundwater identified as the main speleogenetic process, coupled locally with sulfuric acid speleogenesis and ghost-rock weathering (Temovski et al. 2013; Temovski 2016). The insight obtained from this groundwater geochemical study was then used to provide an improved conceptual model of the hypogene karst system.
Research area
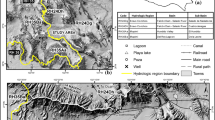
The study sites are located in the southern parts of the Republic of North Macedonia, in Mariovo, a hilly to mountainous area deeply incised by the valleys of Crna Reka and its tributaries (Fig. 1). The area spreads on both sides of the contact between the Vardar Zone and the Pelagonian Massif. These major tectonic zones are overlain by structures formed within the extensional tectonics of the South Balkan Extensional System (Burchfiel et al. 2008; Dumurdžanov et al. 2005), mainly the Mariovo Basin, bounded to the south by the mountainous terrain of the Kožuf-Kozjak volcanic centers (also known as Voras in Greece).
Location and geological setting of the study area. The approximate boundary between the Pelagonian Massif (PM) and the Vardar Zone (VZ) is also indicated. The potential recharge areas for different springs are also indicated: Gugjakovo Springs and Karši Podot – cyan outline; Melnica Spring – cyan crosshatch; Manastir – grey crosshatch; Toplek 2 and Toplek 4 – black crosshatch
Karst is developed here in marble of supposedly Precambrian and Cambrian age, as well as in Cretaceous and Triassic limestone and dolomite, and varieties of Pliocene-Quaternary travertine. The Precambrian and Cambrian age of the Pelagonian marble formations has been recently questioned, assigning them Triassic to Jurassic age based on preliminary local 87Sr/86Sr measurements and various studies of its continuation to the south in Greece (Most 2003). The main structures are mainly spread in the NNW–SSE direction, with the carbonate rocks part of a series of nappe structures, except for the travertine deposits which are found either within, or topping, the Pliocene-Quaternary sedimentary sequences, or on Pleistocene river terraces. This long NNW–SSE karst stripe is cut transversely by the gorge-like valleys of Crna Reka (at Podot) and its tributary Buturica (at Melnica), where the three low-temperature (warm) thermal karst springs, that are the main study sites, are found (Fig. 1).
Hypogene karstification in the area has been characterized on the basis of cave morphology and cave deposits (Temovski 2016). The main speleogenetic mechanism is hydrothermal speleogenesis, i.e. dissolution of carbonate rock due to cooling of CO2-rich thermal water, with increased geothermal gradient attributed to the Neogene-Quaternary Kožuf-Kozjak volcanism. At places, due to local geological or lithological control, this is coupled with other hypogenic processes such as sulfuric acid speleogenesis (Temovski et al. 2013, 2018) or ghost-rock weathering, i.e. preferential dissolution of calcite due to cooling of thermal waters, leaving in-situ dolomitic sand residue (Temovski 2016, 2017).
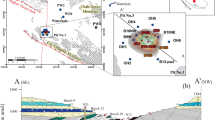
Melnica spring is located at the riverbed in the small gorge of Buturica Valley, with groundwater discharging from calcite marble along a lens of schists (Fig. 2). It is located 100 m below Provalata Cave, a fossil hypogene cave where both CO2-based hydrothermal and sulfuric acid speleogenesis were identified (Temovski et al. 2013, 2018). In Crna Reka, at Podot locality (Figs. 1 and 3), the active thermal cave Karši Podot is developed in dolomite marble on the right valley side, and on a 3-m high river terrace on the opposite side of the river, the lukewarm Gugjakovo springs discharge from travertine deposits.
Preliminary findings from a previous sampling campaign in 2016 (Temovski and Palcsu 2018) indicated that the water at these springs is a mixture of cold and thermal groundwater, with possible interaction with the Kožuf-Kozjak volcanics.
In addition to these, three more springs were also included: Manastir, Toplek 2 and Toplek 4. Although they do not belong to the same groundwater systems, they were added as comparative sites, representing either a young fresh groundwater system (e.g. Manastir and Toplek 4) or a low-temperature thermal groundwater system with similar geochemical composition (e.g. Toplek 2). Manastir is a cold spring, discharging from a large plateau of travertine deposits topping the Mariovo Pliocene-Pleistocene sedimentary sequence. Toplek 2 and Toplek 4 are located further east, on the opposite side of Kozjak Mt, in the foothills of Kožuf Mt (Fig. 1). Toplek 2 has similar temperature to Melnica and Karši Podot, while Toplek 4 is cold and intermittent. Toplek 2 and 4 are located very close to each other and discharge from dolomite rocks, near the Allchar Carlin-type ore complex (Palinkaš et al. 2018). Previous geochemical studies of Toplek 2 (Boev and Lepitkova 2003; Boev and Jančev 2014) show high concentrations of elements characteristic of the ore deposits (e.g. As, Sb, Tl).
Methodology
Methodological approach
As the sampling network for the groundwater systems was restricted to only their springs (no available boreholes), and located in a remote area, with a limited number of sampling locations, the approach relied on the combined use of several geochemical methods.
Radiocarbon (14C) and tritium (3H) combined with noble gases (3H-3He method) were used to identify the mean residence time of the groundwater and to separate young and old components. Water stable isotopes and noble gases were used to identify the source of water and conditions at groundwater recharge. Major ion and trace element composition of the water, as well isotopic composition of the dissolved inorganic carbon (DIC) and dissolved sulfate, were used to interpret the chemical evolution of the groundwater and identify possible interaction with nonkarstic rocks. The concentration and carbon isotope composition of the DIC were used to identify different carbon sources and model the carbon isotope composition of endogenic CO2. The obtained fractions for different carbon sources (soil and endogenic CO2 and carbonate rock) were then used to correct the radiocarbon age of the old groundwater by constraining the contribution of 14C-free carbon. Noble gas concentrations and isotopic composition were also used to identify endogenic noble gases. The applied methodological approach is conceptually illustrated in Fig. 4, showing the use of characteristic geochemical parameters to identify different properties of the groundwater system. Further explanation is also given on the approaches and calculation steps used to identify CO2 sources (section ‘Sources of CO2 and deconvolution of carbon components in DIC’), noble gas recharge temperatures (section ‘Noble gas recharge temperatures’), different groundwater components, and their carbon isotopic composition and mean residence times (sections ‘Calculating the age and fraction of the young groundwater component’, ‘Estimating the carbon isotope composition of the groundwater end-member’, ‘Correction for 14C-free dilution of DIC and calculating the age of the old groundwater’).
Analytical methods
Field sampling was carried out in September 2018 at all locations, except Toplek 4, which was sampled in March 2019 when also Toplek 2 was resampled. Spring water temperature, electrical conductivity (EC) and pH were determined on-site using a Multi 340i multiparameter instrument. Total alkalinity was determined in the field by acidimetric titration, using HCl 0.1 N as titrating agent and methyl orange as indicator. Samples were collected for laboratory analyses to determine water chemistry, stable isotopes, radiocarbon, tritium and dissolved noble gases. Water stable isotope composition, temperature, pH and EC of the three main springs were analyzed more frequently in addition to the main field campaign, when samples for full geochemical analysis were collected. The full list of those measurements is given in the electronic supplementary material (ESM).
Samples for determination of anions were filtered and stored without additional treatment, whereas samples for determination of cations and trace elements were acidified after filtration by addition of suprapure nitric acid. Major anion concentrations were measured on a Metrohm 850 Professional IC, and cations on an Agilent 4100 MP-AES. Major ion charge balance error was less than 5%. Trace element concentrations were measured using Agilent 8800 Triple Quadrupole ICP-MS. Stable isotope analyses of water and DIC were done on an automated GASBENCH II sample preparation device attached to a Thermo Finnigan DeltaPLUS XP mass spectrometer (Vodila et al. 2011). Hydrogen and oxygen isotopes of the water are given as δ2H and δ18O values relative to Vienna Standard Mean Ocean Water (VSMOW), and DIC carbon isotopes are expressed as δ13C values relative to Vienna Pee-Dee Belemnite (VPDB). The precision of the measurements is better than ±0.15‰ for δ18O, ±2‰ for δ2H, and ± 0.08‰ for δ13C. Oxygen and sulfur stable isotope composition of the dissolved SO42− were analyzed with a Thermo Finnigan DeltaPLUS XP mass spectrometer attached to an elemental analyzer (Flash EA). Oxygen and sulfur isotopes of the dissolved SO42− are given as δ18O values relative to VSMOW, and as δ34S values relative to Vienna Canyon Diablo Troilite (VCDT). An individual δ18O and δ34S measurement has a standard deviation (SD) of ±0.3‰ and ± 0.4‰, respectively.
Radiocarbon (14C) analyses of the water samples were done by accelerator mass spectrometry (AMS), following standard procedures (Molnár et al. 2013a), on an EnvironMICADAS 14C AMS facility (Molnár et al. 2013b). The DIC fraction of the water samples was extracted by acid (85% H3PO4) reaction in a vacuum-tight reaction vessel. The produced CO2 gas was cleaned and separated along an on-line gas handling system and graphitized by the sealed tube Zn-based graphitization method (Rinyu et al. 2013). Radiocarbon results are expressed in absolute pMC units (percent Modern Carbon). The water samples for dissolved noble gases were stored in copper tubes sealed by stainless-steel pinch-off clamps. Noble gas measurements were performed by a VG5400 (Fisons Instrument) and a Helix SFT (Thermo Scientific) noble gas mass spectrometers (Papp et al. 2012). Noble gas concentrations are given as cubic centimeters of gas at Standard Temperature and Pressure per gram (cm3 STP/g). Tritium (3H) concentration in water was determined using the 3He ingrowth method (Palcsu et al. 2010). Tritium results are expressed as tritium units (TU), with precision of the measurements better than 0.1 TU.
All analytical measurements were done at the Isotope Climatology and Environmental Research Centre, Institute for Nuclear Research, Debrecen, Hungary.
The distribution of aqueous carbon species and saturation indices for aragonite, calcite and dolomite, as well as pCO2, were calculated using PHREEQC Version 3 software (Parkhurst and Appelo 2013). Statistical analyses of chemical parameters were done with IMB SPSS Statistics v.23 using normalized values.
Sources of CO2 and deconvolution of carbon components in DIC
To identify the sources of CO2 the applied approach first separates the amount of carbon contributed to the DIC by dissolution of carbonates (Crock) from the external carbon (Cext), i.e. carbon from CO2 not accounted for by dissolution of carbonate rocks (Chiodini et al. 2000, 2004; Crossey et al. 2009). Crock can be calculated from the water chemistry data, as Crock = Ca2+ + Mg2+ − SO42−, where the amount of Ca and Mg derived from dissolution of carbonate rocks is corrected for dissolution of sulfates (Chiodini et al. 2000). Cext is then calculated as Cext = DIC – Crock. The δ13C of the external carbon can be estimated from the following equation (Chiodini et al. 2000): (δ13Cext × Cext) = (δ13CDIC × DIC) − (δ13Crock × Crock). For δ13Crock the average values of measured samples of the carbonate rocks from the studied aquifers were used (Fig. 5).
The obtained δ13Cext ranged from values characteristic of soil-derived CO2, to values reflecting mixture of soil CO2 and variable amounts of endogenic CO2. To estimate the δ13C of the endogenic CO2, the mixing of a soil end-member (with δ13C defined by samples with δ13Cext reflecting soil-derived CO2 and a range of values for Cext), with an endogenic end-member was modeled. The δ13C value of the endogenic end-member was constrained by the best fit mixing line between Cext and δ13Cext values of springs that are located next to each other, which, based on their geochemical composition and proximity, represent mixtures with variable contribution of the same end-members, and as such, they should fall on the same mixing line (section ‘Stable isotope composition of DIC and the source of CO2’). The fractions of carbon in DIC derived from soil CO2 (fsoil) and endogenic CO2 (fendogenic) at each spring can then be calculated from the binary mixing model using the equation: fsoil = [(δ13Cext - δ13Cendogenic)/(δ13Csoil - δ13Cendogenic)]/ fext, where δ13Csoil is the soil end-member value, δ13Cendo is the modeled endogenic end-member value, fext is the fraction of external carbon in DIC calculated as fext = Cext/DIC and fendogenic = 1 – fsoil. The carbon in DIC derived from dissolution of carbonate rocks (frock) can be calculated as frock = Crock/DIC, and the total carbon balance is expressed as DIC = fsoil + fendogenic + frock = 1.
Noble gas recharge temperatures
The noble gas recharge temperatures (NGTs) reflect the temperature during recharge at which the groundwater was equilibrated to the soil air noble gas concentrations (Aeschbach-Hertig and Solomon 2013). The NGTs were estimated by using all noble gases except He, which usually has higher concentration due to contribution from other sources (crustal, mantle). The noble gas concentration in air, besides the temperature, depends also on air pressure, which varies with elevation. An estimation on the recharge elevation was made by extracting the mean elevations from a digital elevation model (DEM) for the outcrops of the carbonate formations likely contributing to these aquifers (Fig. 1). NGT values were calculated using the NobleBook excel worksheet (Aeschbach-Hertig et al. 2000, 2008). As the recharge elevations are an average value, the estimated NGTs are also reflecting an average recharge temperature of water infiltrated at supposedly different elevations. The uncertainty in the NGTs due to recharge elevation uncertainty (taken as 1SD from mean elevations) ranged from 0.4 to 1.6 °C, which is 4–11% of the calculated NGT value.
Calculating the age and fraction of the young groundwater component
The age of the young groundwater cannot be determined based only on 3H data, due to possible 3H contribution from the thermonuclear testing in the 1950–1960s (bomb peak 3H). By combining 3H and noble gas data, based on the accumulation of 3He (3Hetrit) from the decay of 3H (half-life of 12.32 years), an apparent (3H-3He) age (expressed in years) can be calculated using the decay formula t = −17.77 × ln[3H/(3H + 3Hetrit)], which represents the period since the 3H-bearing water reached the groundwater (Schlosser et al. 1988). 3H in the water recharging the aquifer before the thermonuclear testing, i.e. older than 1953 (assuming 4 TU for 3H in precipitation), would have decayed by the sampling time (2018) to a value of <0.1 TU. When plotted on a graph showing the 3H in precipitation decayed to the sampling time, samples with >0.1 TU that are below the precipitation curve represent mixture of old 3H-free groundwater and young groundwater. By using the 3H-3He apparent ages to constrain the recharge period of the young component, the fraction of the young groundwater can be estimated from the measured 3H concentration of the groundwater and the expected recharge 3H concentration obtained from the precipitation 3H dataset for the given recharge period, decayed to the sampling time. For the precipitation 3H concentrations, as there are no historical data available for the area, the data from the Vienna GNIP (Global Network of Isotopes in Precipitation) station was used, both monthly and yearly datasets.
Estimating the carbon isotope composition of the groundwater end-members
If it is assumed that the young groundwater DIC evolved due to carbonate dissolution under a system closed to soil CO2, then the 14C of the young component can be calculated from 14Cyoung = 0.5 × (14CCO2 + 14Crock) (Han and Plummer 2016), i.e. half of the 14C of the soil equilibrated CO2, as 14Crock is 0 pMC. The 14C of the soil equilibrated CO2 can be estimated from the 14C of the atmospheric CO2 (Hua et al. 2013) for the given recharge period based on the 3H-3He age. By using springs that represent mixtures with variable contribution of the same end-members for the young and old groundwater (e.g. Gugjakovo and Karši Podot in this case), the δ13C of the young component can be calculated from the linear regression of their δ13C and 14C values (14C = a + b × δ13C) and the known 14C value for the young component (i.e. δ13Cyoung = (14Cyoung – a)/b; Fig. 6). The carbon isotope composition (δ13C and 14C) of the old groundwater can then be calculated from the composition of one of the springs and the young groundwater by using a binary mixing model [Cmix = fyoung × Cyoung + (1 - fyoung) × Cold] and the fraction of the young component (fyoung) for selected spring estimated from 3H (Fig. 6).
Correction for 14C-free dilution of DIC and calculating the age of the old groundwater
The radiocarbon content of the DIC can be used to estimate the mean residence time of the old groundwater component, assuming that the lowering of DIC 14C content along the groundwater flow-path is only due to 14C decay (half-life of 5,730 years). However, there can be a number of processes and mechanisms along the flow-path (e.g. carbonate rock dissolution, methanogenesis, endogenic CO2, mixing with young groundwater etc.) that can alter the 14C content of DIC, masking the decay signal (Clark 2015; Han and Plummer 2016). In epigene karst groundwater systems, carbonate dissolution is the major modifying factor, diluting the soil 14C signature by addition of 14C-free (dead) carbon, as this is the main process that controls the formation of the system (Ford and Williams 2007). In hypogene karst systems, this can be further complicated by the presence of various endogenic gases, that can contribute dead carbon to the DIC pool, especially CO2 of metamorphic or magmatic origin (e.g. Bayari et al. 2009a; Clark et al. 1989). To correct for the 14C dilution, a number of methods and approaches have been developed (see Han and Plummer 2016 for a recent review). Most of them are based on the mass balance of DIC carbon species or carbon isotopes and determine a radiocarbon dilution factor q, that represents the fraction of the initial 14C at recharge (e.g. Ingerson and Pearson 1964; Tamers 1975; Fontes and Garnier 1979; Mook 1980). Their applicability is constrained by the assumed chemical reactions and isotopic fractionation along the evolution of the DIC carbon composition (Han and Plummer 2016). Some of them are based on a statistical approach and rely on the use of a larger number of samples from the same groundwater system (e.g. Gonfiantini and Zuppi 2003), or they apply geochemical modeling for the evolution of 14C along the flow-path of the groundwater system (e.g. Plummer et al. 1994; Bayari et al. 2009a). Mixing with younger groundwater can further complicate the 14C signal, by contributing DIC with higher 14C content due to the thermonuclear testing in the 1950–1960s; thus, in some studies (e.g. Gonfiantini and Zuppi 2003), groundwater samples that indicate mixing with younger (bomb-peak affected) groundwater, are excluded from the determination of the 14C-based mean residence time. However, sometimes the mixed groundwater is the only available option, especially when sampling is limited to spring sites, and can still provide valuable information about the groundwater system.
For the selected study area, the DIC 14C was altered by addition of dead-carbon from dissolution of carbonate rocks, and by endogenic CO2, but also by mixing with younger groundwater (section ‘Radiocarbon and the mean residence time of the old groundwater’). To correct for this, the used approach was based on the previously deconvoluted components of carbon derived from dissolution of soil CO2, carbonate rock and endogenic CO2 (section ‘Sources of CO2 and deconvolution of carbon components in DIC’). The soil carbon component of the old groundwater (fsoil-old) can be calculated from the young groundwater fraction (fyoung), given that the endogenic component is provided solely by the old groundwater and the young groundwater has half of the carbon from the bedrock and half from the soil (closed system carbonate dissolution), i.e. fold = (frock – 0.5fyoung) + (fsoil – 0.5fyoung) + fendogenic. The soil fraction in the old groundwater is then calculated from fsoil-old = (fsoil – 0.5fyoung)/fold, and represents a different derivation of the dilution factor q, that also considers contribution of dead carbon from endogenic CO2. The radiocarbon age of the old component (expressed in years) can then be calculated from the decay equation t = −8267 × ln[14Cold/(q × 14C0], where 14Cold is the old groundwater 14C composition and 14C0 is the initial soil 14C at recharge, taken as 100 pMC.
Results
Chemical characterization of the groundwater
All studied springs, except for Toplek 4, have relatively stable temperature, electrical conductivity (EC) and pH; these are parameters that were measured more frequently (see the ESM). Melnica and Karši Podot have the highest average temperature (22–23 °C), well above the mean annual air temperature (MAAT) for the area (11–12 °C; Temovski 2016), with Gugjakovo somewhat cooler (17.5 °C) but still above MAAT. Manastir also appears slightly above MAAT (14.2 °C), whereas Toplek 4 is the coldest (7.1 °C) and Toplek 2 has similar temperature (21 °C) to Karši Podot and Melnica. Average pH values range from ~6.5 at Karši Podot and Melnica, to near neutral at Gugjakovo (6.9), Manastir (7.1) and Toplek 2 (7.3), and are highest at Toplek 4 (8.3).
All of them have similar chemical composition (Ca-HCO3 type; Fig. 7), with typical karst water major-ion concentrations (Ca2+ > Mg2+ > Na+ > K+, and HCO3− > SO42− > Cl−). Only Toplek 2 has somewhat higher SO4 concentrations (Table 1). EC values range from 144 μS/cm at Toplek 4 to ~1,000 μS/cm at Melnica. The springs have similar major ion composition, with Ca2+ as the most abundant cation (Table 1). Alkalinity ranges from 81.4 mg/L at Toplek 4 up to 633 mg/L at Melnica. Cl− (up to 13.2 mg/L) and NO3− (up to 13.9 mg/L) concentrations are relatively low, with the highest Cl− content at Toplek 2 and the highest NO3− content at Manastir, likely reflecting some local contamination. SO42− concentrations range from 3.3 mg/L (Toplek 4) up to 34.1 mg/L at Toplek 2. Ca2+ ranges from 18.9 mg/L at Toplek 4, up to 157 mg/L at Melnica and Karši Podot. Mg2+ is highest at Karši Podot (28 mg/L) and lowest at Manastir (2.6 mg/L). Na+ and K+ are in relatively low concentrations.
HCO3 is the dominant DIC component, with higher CO2 concentrations found at Gugjakovo, Karši Podot and Melnica. Calculated pCO2 values at Manastir, Toplek 2 and Toplek 4 are lower than 10–1.7 atm (Table 2) and within the range for soil derived CO2 (10–2.5 to 10–1.5 atm; Clark 2015). pCO2 at Melnica and Karši Podot (~10–0.6 atm) is higher than expected for soil CO2 indicating a different source for CO2. Gugjakovo has an intermediate value (10–1.2 atm) that might also indicate mixing of CO2 from different sources. All of the springs are undersaturated with calcite, aragonite and dolomite, although Gugjakovo, Toplek 4 and Melnica have saturation index (SI) values approaching saturation with calcite (Table 2; Fig. 7). Manastir has the lowest SI values for dolomite, reflecting the travertine aquifer lithology, while Toplek 2 and Gugjakovo have the highest SI values for dolomite, reflecting dominantly interaction with dolomite bedrock.
Mg/Ca molar ratios range between 0.06 and 0.66 and indicate dissolution of both dolomite and calcite at all locations, except at Manastir (Mg/Ca = 0.06), where only calcite dissolution is indicated, reflecting travertine aquifer lithology (Table 2; Fig.7). At Melnica, water discharges from calcite marble, thus the increased Mg/Ca ratio (0.27) indicates that at depth water likely circulates either within or at least at the contact with dolomite. Karši Podot is discharging from dolomite marble, but has similar Mg/Ca ratio (0.29) compared to Melnica, indicating either preferable calcite dissolution, or also circulation through calcite marble at depth. A higher Mg/Ca ratio at Gugjakovo Springs indicates dissolution of dolomite marble. The Mg/Ca ratios of Toplek 2 (~0.66) and Toplek 4 (0.36) reflect their dolomite aquifers lithology, with higher Mg/Ca ratios at Toplek 2 likely reflecting the longer water–rock interaction.
The studied springs have higher concentrations of certain trace elements (TE), of which boron, strontium, arsenic, and lithium are the most abundant (Table 3; ESM). Melnica and Karši Podot have similar TE compositions, with Melnica having slightly higher concentrations. Gugjakovo also has similar composition to Melnica and Karši Podot but with much lower concentrations. Boron concentrations are highest at Melnica (705 μg/L), Karši Podot (462 μg/L) and Gugjakovo, and are much lower (<15 μg/L) at the other springs. Arsenic is highest at Toplek 2 (414 μg/L), but Melnica (209 μg/L) and Karši Podot (149 μg/L) have also high concentrations. High arsenic concentrations have been reported previously for Toplek 2 (Boev and Lepitkova 2003; Boev and Jančev 2014). Toplek 2 is located next to the Allchar As-Sb-Tl-rich ore deposit (Palinkaš et al. 2018), where the trace element composition was attributed to higher water–rock interaction with the volcanic rocks of the Kožuf-Kozjak volcanism. Lithium is increased at Melnica, Karši Podot and Gugjakovo (35–51 μg/L), with much lower concentrations (<3 μg/L) found at the other springs. Higher concentrations of boron and lithium found at Melnica, Karši Podot and Gugjakovo, but not at the Toplek springs, might be due to contact at depth with the granitoid plutons of the Pelagonian massif. Strontium is generally increased at all springs, except Toplek 4, with concentrations ranging from 82 to 348 μg/L, and Melnica having highest concentration.
Pearson’s correlation coefficients of the physico-chemical parameters show several highly correlated parameters (Table 4; ESM), which reflect water–rock interactions with three main aquifer lithologies. HCO3, Ca, and Mg are reflecting the dominant dissolution of carbonate rocks. Na and K likely reflect water–rock interactions with the various schist formations or the Pelagonian metamorphic basement. Strong correlation of B, U, Cs, Rb and Sr points to contact with the granitoid bodies within the Pelagonian basement. The highly correlated As, Mo, Sb and Tl clearly reflect contact with the Kožuf-Kozjak volcanic complex or ore deposits related with earlier hydrothermal system connected to the volcanism.
This is further illustrated by the hierarchical cluster analysis (HCA) which reflects the similarities between the studied groundwater (Fig. 8). The clustering of the chemical parameters reveals distinct groups, and in addition to the circulation through the carbonate formations, indicates also interaction with rocks from the Pelagonian basement, and rocks of the Neogene-Quaternary Kožuf-Kozjak volcanic complex.
Water stable isotope composition
Water stable isotope composition was measured more frequently and the springs showed relatively small variation in δ18O and δ2H with values that fall near the local and global meteoric water lines (Fig. 9; ESM). From the main studied springs, Gugjakovo, Karši Podot and Melnica have similar values, of which Gugjakovo has the lowest (−10.4 ± 0.5‰ δ18O, −72.2 ± 2.5‰ δ2H) and Karši Podot (−10.1 ± 0.3‰ δ18O, −70.0 ± 2.1‰ δ2H) and Melnica (−10.0 ± 0.3‰ δ18O, −66.9 ± 1.5‰ δ2H) have slightly higher values. Manastir has more positive values then them (−9.2 ± 0.4‰ δ18O, −65.4 ± 1.1‰ δ2H), while Toplek 2 (−9.9 ± 0.2‰ δ18O, −63.6 ± 1.3‰ δ2H) and Toplek 4 (−10.6 ± 1.1‰ δ18O, −69.5 ± 7.3‰ δ2H) located further east, have similar composition, with Toplek 4 having high variability and reflecting recent recharge and fast circulation.
Water stable isotope composition of the springs. Sample codes same as in Fig. 7
They all have lower values compared to the recently measured values for the mean annual composition of the local precipitation (MAP; −8.9‰ δ18O and −58.5‰ δ2H; M. Temovski, Institute for Nuclear Research, Debrecen, unpublished data; Fig. 9). This can be a result of dominant recharge of the aquifers during the cooler period of the year or higher contribution from water infiltrated at higher elevation, or, for the older groundwater, it might indicate recharge under cooler climate conditions.
Stable isotope composition of the dissolved sulfate
The sulfur stable isotope composition of the dissolved SO42− was analysed with attempts also to measure the oxygen stable isotopes. Melnica and Karši Podot had similar values for δ34S (−1.3 and −1.4‰), Toplek 2 had lowest values (−4.0‰), while positive δ34S values were found at Manastir (+2.1‰) and Gugjakovo (+5.1‰). δ18O values were positive and ranged from +0.2‰ at Toplek 2 up to +5.6‰ at Karši Podot (Table 5). The sulfate stable isotopes at Melnica, Karši Podot and Toplek 2 are within the range of values (−7.5 to +0.7‰ δ34S; −3.9 to +8.2‰ δ18O) found in the gypsum deposits of Provalata Cave (Temovski et al. 2018), whose origin is attributed to oxidation of H2S in vadose settings (i.e. sulfuric acid speleogenesis by condensation corrosion). Their δ34S values are also within the range (−9.8 to 0.9‰) of the sulfide minerals found in Allchar ore deposit, where contribution of magmatic H2S was identified for the main mineralization stage (Palinkaš et al. 2018). The source of the dissolved sulfate at these springs, thus can be attributed to oxidation of deep-seated sulfides (either H2S or sulfide minerals deposited by earlier hydrothermal systems). The values at Gugjakovo and Manastir indicate a different source, likely oxidation of sedimentary pyrite.
Stable isotope composition of DIC and the source of CO2
The δ13CDIC showed clear differences between the springs (Table 6), with Melnica (−0.93‰) and Karši Podot (−1.95‰) having the highest values, Gugjakovo (−7.34‰) and Toplek 2 (−7.87‰) somewhat intermediate, and lowest values found at Manastir (−9.63‰) and Toplek 4 (−9.25‰).
The pCO2, pH and δ13CDIC at Manastir and Toplek 2 indicate DIC evolution by carbonate dissolution mostly under closed system conditions after some initial open system dissolution. The very low pCO2 (10–3.5 atm), high pH and low alkalinity at Toplek 4 indicate closed system carbonate dissolution. The high pCO2, low pH and high δ13C at Melnica and Karši Podot indicate a possible contribution of endogenic CO2.
The obtained values for δ13Cext range from −20.8 to −2.3‰ (Table 6). Values reflecting soil δ13CCO2 are found at Manastir and Toplek 4 (−20.1 ± 0.7‰), reflecting the dominantly C3 vegetation of the area (mostly covered by forests), and the others show variable contribution of endogenic CO2. To estimate the δ13C of the endogenic CO2, the mixing of a soil end-member with δ13C of −20.1‰ and a range of 0.5–5 mmol/L for Cext, with an endogenic end-member was modeled (Fig. 6). The Cext and δ13Cext values of Karši Podot and Gugjakovo were used to constrain the δ13C of the endogenic end-member, as their close proximity and similar geochemical composition indicate that they share the same end-members. The closest match was found for a mixing line defined by soil CO2 with δ13C of −20.1‰ and Cext of 4.9 mmol/L to an endogenic end-member with δ13C value of +4.5‰ (Fig. 10). Such δ13C value indicates a metamorphic origin for the endogenic CO2 component (Chiodini et al. 2000; Clark 2015). However, based on the noble gas data (section ‘Noble gases, tritium and the mean residence time of the young groundwater’), there is some contribution of mantle helium, indicating also possible contribution of mantle CO2, thus the metamorphic CO2 δ13C might be somewhat underestimated. Based on the three identified sources of carbon (carbonate rock, soil CO2 and endogenic CO2), the fractions of endogenic CO2 range from 13% at Toplek 2 and 19% at Gugjakovo, to 51 and 54% at Karši Podot and Melnica, respectively.
a Plot of the amount (Cext) and isotopic composition (δ13Cext) of dissolved carbon from sources other than carbonate dissolution, with studied springs showing variable contribution of endogenic CO2 (δ13C of +4.5‰) to soil CO2 (δ13C of −20.1‰). The open circles represent springs with only soil derived CO2. b The ternary diagram showing the calculated percentages of carbon sourced from dissolution of carbonates (Crock), soil CO2 (Csoil) and endogenic CO2 (Cendo). Sample codes same as in Fig. 7
Noble gases, tritium and the mean residence time of the young groundwater
Noble gas concentrations are close to air-equilibrated values, except for He concentrations, which are somewhat higher at Melnica, and much higher at Gugjakovo (Table 7). The R/Ra ratios (where R and Ra are the 3He/4He ratio of the sample and air, respectively), range between 0.76 and 1.16, with Toplek 4 reflecting air composition (R/Ra ≈ 1), and the others having R/Ra values either lower or higher than 1.
The NGT values range from 3.8 to 22.7 °C (Table 7). The estimated NGT for Manastir (11.8 °C) is within the range of MAAT for the area (Fig. 11a). The NGTs for Gugjakovo, Toplek 2 and Toplek 4 are somewhat lower than expected for the local MAAT at the spring sites, which might be due to different reasons such as infiltration during older cooler periods, larger contribution of water infiltrated at higher elevation, or fast and/or higher contribution from water infiltrated during the winter period. The coldest estimated NGT at Toplek 4 (3.8 °C), clearly reflects winter infiltration, also confirmed by the low spring water temperature, and the recent 3H-3He age. Their δ18O and NGT values follow the relationship of local mean monthly temperatures and monthly precipitation δ18O (as observed during 2018–2019; Fig. 11b), which might be a result of higher contribution of winter infiltration.
Calculated noble gas recharge temperatures, plotted against: a measured spring water temperature; b groundwater δ18O. MAAT is the mean annual temperature at the spring sites, and MAP is the weighted mean annual δ18O in local precipitation. Dashed line (b) shows the relationship of mean monthly air temperature to monthly δ18O in local precipitation (2018–2019)
The estimated NGTs for Karši Podot and Melnica are much higher and clearly reflect the temperature of the groundwater at the spring (Fig. 11a). At Karši Podot, this is probably due to re-equilibration of the dissolved noble gases to the cave atmosphere behind the point where the water emerges at the cave wall. Considering the sponge-like morphology of the cave (Temovski 2016), there is likely a large contact of air with the water table along many small interconnected cavities. Similarly, at Melnica, a close match of the NGT to the spring water temperature might indicate an existence of a cave passage behind the spring where the water table is in contact with a large cave air mass.
3H was found in all of the springs and ranged from 1.5 to 5.8 TU. Karši Podot (1.5 TU) and Melnica (1.7 TU) have the lowest values, Toplek 2 (2.3 TU) and Manastir (2.7 TU) intermediate, and Gugjakovo (3.4 TU) and Toplek 4 (5.8 TU) had the highest 3H values. Assuming no mantle helium, and after removal of air-derived and terrigenic (crustal) He, the calculated concentrations of 3He formed by 3H decay (3Hetrit), except for Melnica and Gugjakovo, range from 0 to 8.3 × 10−14 cm3 STP/g, yielding 3H-3He apparent ages of 0 ± 3 years for Toplek 4, 10 ± 3 years for Manastir, 31 ± 2 years for Karši Podot, and 49 ± 1 years for Toplek 2. The calculated 3Hetrit concentrations were much higher at Melnica (68 × 10−14 cm3 STP/g) and especially at Gugjakovo (909 × 10−14 cm3 STP/g), indicating presence of mantle derived 3He, thus no 3H-3He apparent ages were calculated.
The possible mantle helium contribution can be estimated from a three-component (atmospheric, crustal and mantle helium) mixing model, using R/Ra values corrected for excess air and tritiogenic 3He (Rc/Ra), and the 4He/20Ne ratio. For Melnica and Gugjakovo, considering the high nonatmospheric He concentration, the 3Hetrit contribution is small. Nevertheless, the 3He concentration was corrected based on a 3Hetrit value calculated using the maximum possible 3H input value that can decay to the measured one in the period since the beginning of the thermonuclear testing (taken as 1953). Using 4He/20Ne ratio of 1000 and R/Ra of 8 and 0.02 for the mantle and crustal end-member (Ozima and Podosek 2002), respectively, the calculated mantle He contribution at Melnica is 10% and at Gugjakovo 9%, with also small (2%) mantle contribution found at Karši Podot. Manastir and Toplek 2 have a mixture of crustal and atmospheric helium, while Toplek 4 has only atmospheric helium (Fig. 12).
Helium components in the studied groundwater, corrected for excess air and tritiogenic 3He: a three-component (atmospheric, mantle, crustal) helium mixing plot; b calculated percentages of mantle-, crustal- and air-derived helium. Sample codes (a) same as in Fig. 7
The 3H values of studied springs are lower than the decayed yearly 3H values for Vienna precipitation (Fig. 13). As pointed out, this likely reflects the proportion of the young water fraction, although the actual magnitude could differ due to slightly different values for the local precipitation 3H compared to the used dataset. The mixing of young and old water and their variable fractions are also indicated by other parameters such as the combined presence of 3H with very low values for 14C, and water temperature. However, it should be noted here that the 3H-3He age at Karši Podot might be an overestimation due to contribution of mantle 3He; this is because 2% mantle helium was estimated based on the combined helium and neon isotope data.
Comparing measured spring 3H values with their 3H-3He apparent ages (1σ uncertainty) to historical 3H in precipitation (Vienna GNIP station) decayed to the sampling year of 2018. Sample codes same as in Fig. 7
By using the 3H-3He apparent ages to constrain the recharge period of the young component, for Karši Podot, from the yearly precipitation 3H dataset, 30–47% of young groundwater is found (22.4–128% if the monthly 3H dataset is used), with the maximum value clearly an overestimation (Table 8). For Manastir, the estimated young groundwater contribution is 32–54% based on the yearly data, or 25–80% based on the monthly data. However, this might be an underestimation. Although there are no local data available for the estimated recharge period, recent monitoring (since 2018) of 3H in the local precipitation shows 3H values as low as 4–5 TU for the winter period. If similar values are assumed for the estimated recharge period (2005–2012), then the 3H value at Manastir can be explained by 3H decay from precipitation infiltrated dominantly during the winter period, thus the water represents only young groundwater. Toplek 2 is much lower than the precipitation curve, based on which the estimated young groundwater contribution is 13–19% (yearly data) or 10–35% (monthly data).
Radiocarbon and the mean residence time of the old groundwater
The measured DIC 14C concentrations (14CDIC) range from 9.7 to 71.4 pMC, and also show three groups of values: Melnica (9.7 pMC) and Karši Podot (15.3 pMC) have the lowest, Toplek 2 (48.5 pMC) and Gugjakovo (48.9 pMC) intermediate, and Toplek 4 (68.4) and Manastir (71.4) the highest concentrations (Table 9; Fig. 14a). Based on their 3H-3He age and carbon isotope composition, Manastir and Toplek 4 are considered as modern waters, with their 14C values diluted by carbonate dissolution under a system mostly closed to soil CO2. The waters at Melnica, Karši Podot and Gugjakovo represent a mixture of young and old groundwater, and from their geochemical composition it is evident that Gugjakovo has the highest and Melnica the lowest contribution of young groundwater.
Relationship of δ13C and 14C in DIC: a all samples; b derivation of the young and old groundwater carbon isotope composition at Podot locality. Sample codes same as in Fig. 7
As discussed before, Karši Podot and Gugjakovo likely represent mixtures with variable contributions of the same end-members for the young and old groundwater. Furthermore, from the 3H-3He age, the recharge period for the young component (1986–1989) is known, for which the 14C of the atmospheric CO2 (Hua et al. 2013) was between 116 and 119 pMC (mean 117.5 pMC). Using the approach detailed in section ‘Estimating the carbon isotope composition of the groundwater end-member’’, the calculated carbon isotope composition of the young component is 58.8 pMC 14C and − 8.9‰ δ13C (Fig. 14b). The old groundwater at Karši Podot and Gugjakovo (Podot locality), calculated from the carbon isotope composition of the young groundwater and Karši Podot and the estimated young fraction at Karši Podot, thus has 14C of 2.7 pMC and δ13C of 0.07‰, reflecting the large contribution of endogenic CO2. From the binary mixing model and the estimated compositions of the end-members, 82% contribution of the young groundwater can be found at Gugjakovo.
To calculate a radiocarbon age for the old component at Podot (Gugjakovo and Karši Podot), first a correction has to be made for the dilution of the soil 14C signature by addition of 14C-free (dead) carbon from dissolution of carbonate rock and endogenic CO2. Using the deconvoluted components of carbon in the old groundwater (section ‘Stable isotope composition of DIC and the source of CO2’; Table 6), and the approach detailed in section ‘Correction for 14C-free dilution of DIC and calculating the age of the old groundwater’, a dilution factor of 0.17 can be calculated, from which a conventional 14C age of 15.3 ka is obtained for the old groundwater at Karši Podot and Gugjakovo (Table 9).
For Melnica there is no separate estimate for the young fraction, so the same approach cannot be used to calculate the carbon isotope composition of the old component. However, the Melnica sample is very close to the δ13C-14C mixing line of Podot locality (Fig. 14b), indicating that the old component at Melnica will not be significantly different. If no young groundwater contribution is assumed, the dilution factor q will be represented by the total of soil derived carbon (0.20), and from the measured 14C of 9.7 pMC a 14C age of 6.1 ka can be calculated. This should be considered as a minimum age, as young groundwater contribution is clearly indicated by the presence of 3H. The 14C age of 15.3 ka obtained for Podot locality thus should be considered as a maximum age for Melnica. Using the carbon composition of the young end-member at Podot locality, a maximum contribution of 12.8% young groundwater can be calculated for Melnica, as well as an old end-member composition of 2.5 pMC 14C and 0.24‰ δ13C (Table 9).
Based on the 3H data Toplek 2 also appears to be a mixture of old groundwater with 10–35% young groundwater. The δ13C of −9.3‰ at Toplek 4 can be considered as a representative value for the young component evolved under closed system carbonate dissolution. From this value and the fractions for the young component, a δ13C value of −7.4 ± 0.3‰ can be calculated for the old groundwater. The 14C composition of the young component at Toplek 2, should be higher than the one of Toplek 4 as, based on the 3H-3He age, it must have been affected by bomb-derived 14C. For the estimated recharge period (1969–1971) the atmospheric 14C was 151–156 pMC (mean 153.5 pMC), and with half of that (closed system dissolution) as the young component 14C composition, the old groundwater 14C at Toplek 2 should have 38.7 ± 6.8 pMC. The dilution factor q at Toplek, calculated from the carbon components is 0.46, using which a 14C age of 1.5 ± 1.5 ka can be calculated (Table 9). The large age error at Toplek 2 is due to the large error in the estimate for the contribution of the young groundwater.
Geothermometry
An attempt was made to estimate the reservoir temperatures of the warmest springs using different geothermometers based on: SiO2 (Fournier 1977), Na-K-Ca (Fournier and Truesdell 1973) with corrections for Mg (Fournier and Potter 1979) and CO2 (Paceš 1975), as well as K-Mg (Giggenbach 1988) and δ18OSO4-H2O (McKenzie and Truesdell 1977). The estimated reservoir temperatures range from 60 to 175 °C (Table 10). The most comparable temperature estimates are given by the SiO2, K-Mg and Na-K-Ca (pCO2 corrected) geothermometers, with values of 60–65 °C for Karši Podot, 60–71 °C for Melnica and 64–88 °C for Toplek 2. These estimates are considered here as minimum temperatures, as the waters were diluted by mixing with 10–20% cold water, although the cold water has likely much lower dissolved content.
Palinkaš et al. (2018) estimated temperatures from ~100 °C to more than 200 °C for the hydrothermal fluids that carried the mineralization of Au, As, Sb and Tl at Allchar. Considering this, 100 °C can be considered as an upper limit for the reservoir temperature of these groundwater systems. The lower estimated reservoir temperatures than the earlier hydrothermal systems likely reflect the later stage of the thermal evolution of these systems. This is also supported by the estimated temperature of <50 °C for the older phase (>1.5 Ma) of calcite deposition in Provalata Cave (located above Melnica Spring), that acted as a feeder to a former spring (Temovski et al. 2013; Temovski 2016).
Geothermal gradients are not well constrained, with reported values of more than 40 °C/km for the Vardar Zone (Kotevski 1987). Using a geothermal gradient estimate of 40 °C/km, the circulation depths are ranging from 0.9–1 km at Karši Podot, 0.9–1.2 km at Melnica and 1.1–1.7 km at Toplek 2. However, the geothermal gradient in this area is also likely underestimated (especially at Toplek locality), thus both the reservoir temperature and the circulation depth are considered as a loose approximation.
Discussion
Estimation of groundwater component fractions by combined use of 14C, δ13C, 3H and noble gases
As indicated by several parameters in their geochemical composition (e.g. temperature (T), EC, TDS, 3H, 14C, δ13C), the studied spring waters at Melnica, Karši Podot and Gugjakovo represent mixtures of two end-members, a young fresh groundwater and an old thermal groundwater. Without clear end-member representatives at the studied locations, the approach to estimate their compositions is based on one main assumption: the selected springs (Karši Podot and Gugjakovo) share the same end-members, i.e. they are mixtures of different proportions from the same groundwater end-members. As indicated by their close proximity and especially their geochemical composition, this assumption seems valid for the two selected springs, with Karši Podot having higher contribution of the old groundwater (e.g. higher T, EC, TDS, δ13C and trace element concentrations, and lower 3H and 14C) and Gugjakovo having higher contribution of the young groundwater (e.g. lower T, EC, TDS, δ13C and trace element concentrations, and higher 3H and 14C). The fraction of the young component was estimated based on combined use of the spring water 3H concentration and 3H-3He apparent age (young component recharge period), by comparison to the historical record of 3H concentration in precipitation decayed to the sampling date. Assuming carbonate rock dissolution under a system closed to soil CO2, the carbon isotopic composition of the young component was estimated from atmospheric CO2 (14C) and the linear relationship of the measured carbon isotope composition at Gugjakovo and Karši Podot (δ13C). To test the reliability of this estimate, the δ13C of the soil equilibrated CO2 from δ13Cyoung = 0.5 × (δ13CCO2 + δ13Crock), assuming closed system carbonate dissolution (Han and Plummer 2016). Using the δ13C value of the young component (−8.9‰) and a value of 2.8‰ for δ13Crock (section ‘Sources of CO2 and deconvolution of carbon components in DIC’), the calculated δ13C of the soil equilibrated CO2 of the young groundwater is −20.7‰. This is close to the values obtained for the soil-derived external CO2 at Manastir and Toplek 4, where closed system carbonate dissolution was found (section ‘Stable isotope composition of DIC and the source of CO2’).
The source of the groundwater
Water stable isotopes, as well as NGTs, clearly show that the groundwater is of meteoric origin. The δ18O and NGT values reflect recharge under cooler temperatures than local MAAT, which, especially for the young shallow groundwater, probably reflects higher infiltration of water during the winter period. This is to be expected in temperate areas, where even though the precipitation amount might be lower in the winter period, higher evapotranspiration in the summer period prevents larger infiltration (e.g. Jasechko et al. 2014). However, at Karši Podot and Melnica, where the contribution of shallow young groundwater is small, even though the NGTs are not reflecting recharge values, lower δ18O and δ2H values might reflect lower MAAT values, which could be as a result of recharge of the old groundwater at cooler climate conditions, or considering the hydrogeological settings, recharge at higher elevations, or both.
Water–rock interactions
Although the trace element composition indicates interaction with volcanic rocks of Kožuf-Kozjak volcanic system, as well as metamorphic and magmatic rocks of the Pelagonian basement, the major ion composition clearly reflects the carbonate aquifer lithology. Mg/Ca molar ratios indicate dissolution of both calcite and dolomite minerals at Melnica, Karši Podot and Gugjakovo springs. The Mg/Ca ratio at Melnica, discharging from calcite marble, indicates also flow through the dolomite marble formation, which is quite plausible considering the thermally altered parts found in the nearby dolomite marble formation north from Melnica (Fig. 2; Temovski 2016). At Karši Podot the Mg/Ca ratio is similar, although the water is discharging from dolomite marble. This could be due to incongruent dissolution within the dolomite formation, as such a process has been identified in Karši Podot Cave. Within the dolomite marble, the porosity was formed by ghost-rock weathering (e.g. Dubois et al. 2014), i.e. preferential dissolution of calcite due to cooling of thermal waters, leaving in-situ dolomitic sand residue (Temovski 2016, 2017). As the slowly moving thermal waters were lacking in sufficient energy to carry away the remaining dolomite sand, the penetrable-size cave passages were formed only when the high-energy back-flooding water of Crna Reka removed the dolomite sand residue. The higher Mg/Ca ratio at Gugjakovo indicates that the source of the fresh water component is the dolomite marble formation to the north, instead of the previously considered Upper Cretaceous limestone formation (Temovski 2016).
The groundwater flow systems
Compared to what is commonly reported for thermal karst groundwater at the discharge areas (Goldscheider et al. 2010), the studied springs generally have lower total dissolved content (<1,000 mg/L) and low values for dissolved SO42− and Cl−. Lukewarm spring waters (<30 °C) of lower salinity discharging at the Buda Thermal Karst (Mádl-Szőnyi and Tóth 2015) have been interpreted as part of an intermediate groundwater flow in unconfined settings, that has somewhat increased Cl− concentration due to mixing along the discharge zone with basinal fluids of a confined, deeper, regional groundwater flow system. Groundwater of lower salinity related to hypogene karst systems is also found in regions where young volcanism is present, generally associated with hydrothermal activity, with higher concentrations of CO2 and/or H2S (Klimchouk 2017). For the Mariovo hypogene karst systems, based on the geochemical composition of the studied springs, mixing of waters from two groundwater flow systems is identified: a young cold freshwater and an older, chemically more evolved thermal water.
The mean residence time of the young shallow groundwater is constrained by the 3H-3He apparent ages, and ranges from recent (<3 years) at Toplek 4, ~10 years at Manastir, ~30 years at Podot locality and ~50 years at Toplek 2. Manastir and Toplek 4 have the youngest groundwater, which reflects the relatively fast groundwater flow of the local groundwater systems developed in highly permeable travertine and dolomite of higher permeability, respectively. The young groundwater at Podot and Toplek is somewhat older, and reflects either slower groundwater flow of local groundwater systems in less permeable marble and dolomite rocks, or possibly (e.g. in the north stretching marble stipe at Podot) an intermediate groundwater flow system.
The 14C age of the old groundwater component at Podot, if based on the calculated carbon isotope composition (~30 ka), is clearly overestimated. This is due to addition of 14C-free carbon, partly due to dissolution of carbonate rock, but more importantly due to the large contribution (up to 54% of total DIC) of endogenic CO2 (Table 6). Nevertheless, the dead-carbon dilution-corrected 14C age at Podot (~15 ka) still reflects long mean residence time of the old groundwater, with the estimated mean residence time at Melnica between ~6 and ~15 ka. The circulation depth of 0.9–1.2 km at Melnica and Podot indicates deeper circulation of the old groundwater, which can be achieved if the flow is along the dip of the carbonate formation. Considering the topographic position of the discharge areas, the local geological constrains, the estimated circulation depth, the inferred water–rock interactions and the higher recharge elevations, the old groundwater component at Podot represents the discharge of a regional groundwater flow system. The groundwater at Melnica has somewhat lower residence time and represents either a shorter flow path (a subsystem) of the same deeper regional groundwater flow system after reaching a deep fault, or a shallower intermediate groundwater flow system. However, the latter is probably less likely, considering the very similar geochemical properties with the old groundwater at Podot. The very high uncertainty in the 14C age of the old groundwater at Toplek does not allow for any meaningful interpretation.
The hypogene speleogenetic settings
Most of the carbonate rock dissolution likely occurs along the rising limb of the flow in the discharge zone. While increased dissolution here due to mixing with shallower groundwater (i.e. mixing corrosion) is possible, the high dissolved CO2 concentrations are probably of higher importance (Dublyansky 2000). Additionally, cooling due to rising of the thermal groundwater and/or mixing with the cold shallow water, can further increase dissolution due to the retrograde calcite solubility (Andre and Rajaram 2005). These processes are consistent with the findings from Provalata Cave, where the older speleogenetic phase is associated with dissolution of the marble bedrock due to cooling of CO2-rich thermal waters (Temovski et al. 2013). Calcite deposited in isotopic equilibrium with the groundwater DIC at Melnica Spring will have a δ13C of ca. +4‰, which is comparable with the measured high δ13C values (ca. +7‰) in the calcite deposits of Provalata Cave (Temovski et al. 2013). This suggests that this system has operated for a long time, and the abundant travertine deposits in the surroundings (Temovski 2016), deposited in different environments (e.g. lacustrine limestone, paludal limestone, spring tufa), are also likely related to increased alkalinity supplied by these deep groundwater systems.
Mantle helium contribution in the dissolved gases at Podot and Melnica of up to 10% is consistent with the findings on the southern foothill side of Kožuf-Kozjak volcanic system in Greece, where up to 16% mantle helium was reported (Daskalopoulou et al. 2018). H2S oxidation is a likely source of the dissolved sulfate at Melnica and Karši Podot. A magmatic origin of H2S is also possible based on the sulfur isotopic composition, as well as the presence of mantle helium. Mariovo hypogene karst system shows some geochemical similarities to other hypogene karst systems related to young volcanism such as the presence of mantle derived gases, e.g. Sistema Zacatón in Mexico (Gary and Sharp 2006), Mt. Gambier in Australia (Webb et al. 2010) or Konya Basin in Turkey (Bayari et al. 2009b), except that the CO2 at Mariovo is of dominantly metamorphic origin. However, it shares none of the morphological expressions of the discharge zone, with large collapse dolines developed at Sistema Zacatón, Mt. Gambier and Konya Basin, and only relatively small caves found in Mariovo. One reason for this can be the structural difference, with Mariovo hypogene karst developed in highly dipping strata, while the others are developed in sub-horizontal strata, but also the high difference in fluid flux, with Mariovo having both smaller flow rate and gas concentrations.
Conceptual model of the Mariovo hypogene karst system
The results from this geochemical study allow one to put some constraints on the Mariovo hypogene karst system and develop a conceptual model (Fig. 15). The water is of meteoric origin, likely infiltrated along fault structures in the southern parts of the marble stripe. It reached depth of around 1 km, which could be achieved by circulating along fault structures and/or along the dip of the carbonate formation, and then following northward in strike direction. The output zones are at the intersection of low topography and major fault structures at Podot and Melnica localities. Podot locality, is the furthest (~25 km) and lowest output, with water emerging ~15 ka after recharge. Discharge of endogenic gases, mostly metamorphic CO2 and especially some contribution of mantle helium in the output zone indicates deep setting of these fault structures, related to the extensional tectonics of the area. Melnica is likely a subsystem of the same groundwater system, with a shorter flow path (~18 km) and younger age, formed because part of the groundwater was likely forced upward after reaching a deep fault with a higher gas flux.
Most of the carbonate rock dissolution is likely achieved along the rising limb, due to cooling with acidity acquired from endogenic CO2. At depth, closer to the recharge area, the groundwater has contact with rocks from the Kožuf-Kozjak Neogene-Quaternary volcanism. Contact with the underlying metamorphic basement and the granitoid bodies perching it, is also achieved along the northward flow either at depth or along the rising limb in the output zone. In the southern parts, between the recharge zone and Melnica, a schist formation is separating the lower, mostly dolomite marble formation, from the upper, calcite marble formation, partly confining the groundwater system, and forcing the northward deep flow. At the output zones, the deep circulating old water of the regional to intermediate hypogene groundwater system is mixing with the shallow recent water part of the local epigene groundwater systems. This hypogene groundwater system has been active for a long time, as evidenced by the >1.5 Ma age of deposits in Provalata Cave (Temovski et al. 2013). The large travertine deposits found within, or topping, the Pliocene-Quaternary basin sediments near Melnica or Podot are likely related to older stages of the same hypogene karst system.
Conclusion
This study presents an approach whereby various geochemical methods are applied in order to identify different components and properties of groundwater associated with hypogene karst systems. It demonstrates the use of radiogenic and stable isotopes, noble gases and major ion and trace element composition in a case where sampling was restricted only to the spring sites, and in a limited number of locations. In the absence of clearly defined end-member compositions, by combining multiple geochemical methods the groundwater components are identified, as well as their composition and mean residence time, and their geochemical evolution. Furthermore, the carbon sources contributing to the DIC are identified, based on which the carbon isotope composition of the endogenic CO2 is modeled. This is then used to correct the radiocarbon composition for the dilution of the soil 14C signature by addition of 14C-free carbon from dissolution of carbonate rock and endogenic CO2, and calculate a radiocarbon age for the old component.
The obtained results show that the main studied springs represent an output part of a regional hypogene karst groundwater system with a deep-circulating (~1 km), old (~15 ka), thermal (≥60 °C) water, where the old groundwater mixes with young (<50 years), cold (<14 °C) and shallow epigene karst groundwater. These output zones of the hypogene karst system are located at the interception of low topography and deep fault structures along which there is circulation of deep-seated gases, dominantly CO2 of metamorphic origin (+4.5‰ δ13C), with a contribution of mantle helium. The chemical composition of the water indicates interaction at depth with volcanic rocks from the N-Q Kožuf-Kozjak volcanism, as well as with metamorphic rocks and granitoids of the Pelagonian basement. The groundwater geochemistry results of this study complement previous findings based on geochemistry of fossil cave deposits, and confirm that dissolution of carbonate rocks due to cooling of CO2-rich thermal waters is the main hypogene geochemical process in the area.
References
Aeschbach-Hertig W, Solomon DK (2013) Noble gas thermometry in groundwater hydrology. In: Burnard P (ed) The Noble gases as geochemical tracers. Springer, Heidelberg, Germany, pp 81–122
Aeschbach-Hertig W, Peeters F, Beyerle U, Kipfer R (2000) Palaeotemperature reconstruction from noble gases in ground water taking into account equilibration with entrapped air. Nature 405:1040–1044. https://doi.org/10.1038/35016542
Aeschbach-Hertig W, El-Gamal H, Wieser M, Palcsu L (2008) Modeling excess air and degassing in groundwater by equilibrium partitioning with a gas phase. Water Resour Res 44:449–461. https://doi.org/10.1029/2007WR006454
Andre BJ, Rajaram H (2005) Dissolution of limestone fractures by cooling waters: early development of hypogene karst systems. Water Resour Res 41:W01015. https://doi.org/10.1029/2004WR003331
Audra P, Bigot J-Y, Mocochain L (2002) Hypogenic caves in Provence (France): specific features and sediments. Acta Carsol 31(3):33–50. https://doi.org/10.3986/ac.v31i3.378
Bayari CS, Ozyurt NN, Kilani S (2009a) Radiocarbon age distribution of groundwater in the Konya Closed Basin, central Anatolia, Turkey. Hydrogeol J 17:347–365. https://doi.org/10.1007/s10040-008-0358-2
Bayari CS, Pekkan E, Ozyurt NN (2009b) Obruks, as giant collapse dolines caused by hypogenic karstification in central Anatolia, Turkey: analysis of likely formation processes. Hydrogeol J 17:327–345. https://doi.org/10.1007/s10040-008-0351-9
Bayari CS, Özyurt NN, Törk AK, Avci P, Güner IN, Pekkan E (2017) Geodynamic control of hypogene karst development in central Anatolia, Turkey. In: Klimchouk A, Palmer A, De Waele J, Auler A, Audra P (eds) Hypogene karst regions and caves of the world. Cave and karst systems of the world book series, Springer, Cham, Switzerland, pp 449–462, https://doi.org/10.1007/978-3-319-53348-3_27
Boev B, Jančev M (2014) Geochemistry and origine of thermo-mineral waters on Kožuf Mountain. Geol Macedonica 28(2):165–174
Boev B, Lepitkova S (2003) Geohemiski karakteristiki na termomineralnite void na planinata Kožuf [Geochemical characteristics of the thermomineral waters of Kožuf Mt]. Vtoro sovetuvanje za geotermalna energija vo Republika Makedonija [Second Geothermal Energy Conference in the Republic of Macedonia] 2003, pp 61–64
Burchfiel BC, Nakov R, Dumurdžanov N, Papanikolaou D, Tzankov T, Serafimovski T, King RW, Kotzev V, Todosov A, Nurce B (2008) Evolution and dynamics of the Cenozoic tectonics of the South Balkan extensional system. Geosphere 4:919–938. https://doi.org/10.1130/GES00169.1
Chiodini G, Frondini F, Cardellini C, Parello F, Peruzzi L (2000) Rate of diffuse carbon dioxide earth degassing estimated from carbon balance of regional aquifers: the case of central Apennine, Italy. J Geophys Res 105:8423–8434. https://doi.org/10.1029/1999JB900355
Chiodini G, Cardellini C, Amato A, Boshi E, Caliro S, Frondini F, Ventura G (2004) Carbon dioxide earth degassing and seismogenesis in central and southern Italy. Geophys Res Lett 31(7):L07615. https://doi.org/10.1029/2004GL019480
Clark I (2015) Groundwater geochemistry and isotopes. Taylor and Francis, Abingdon, UK
Clark ID, Fritz P, Souther JG (1989) Geochemistry and isotope hydrogeology of the Mount Edziza–Mess Creek geothermal area. Can J Earth Sci 26:1160–1171. https://doi.org/10.1139/e89-099
Crossey L, Karlstrom K, Springer A, Newell D, Hilton D, Fischer T (2009) Degassing of mantle-derived CO2 and he from springs in the southern Colorado plateau region: neotectonic connections and implications for groundwater systems. GSA Bull 121(7–8):1034–1053. https://doi.org/10.1130/B26394.1
Daskalopoulou K, Calabrese S, Grassa F, Kyriakopoulos K, Parello F, Tassi F, D’Alessandro W (2018) Origin of methane and light hydrocarbons in natural fluid emissions: a key study from Greece. Chem Geol 479:286–301. https://doi.org/10.1016/j.chemgeo.2018.01.027
De Waele J, Audra P, Madonia G, Vattano M, Plan L, D’Angeli IM, Bigot J-Y, Nobécourt J-C (2016) Sulfuric acid speleogenesis (SAS) close to the water table: examples from southern France, Austria, and Sicily. Geomorphology 253:452–467. https://doi.org/10.1016/j.geomorph.2015.10.019
Dublyansky YV (2000) Hydrothermal speleogenesis: its settings and peculiar features. In: Klimchouk AB, Ford DC, Palmer AN, Dreybrodt W (eds) Speleogenesis evolution of karst aquifers. National Speleological Society, Huntsville, AL, pp 298–303
Dubois C, Quinif Y, Baele J-M, Barriquand L, Bini A, Bruxelles L, Dandurand G, Havron C, Kaufmann O, Lans B, Maire R, Martin J, Rodet J, Rowberry MD, Tognini P, Vergari A (2014) The process of ghost-rock karstification and its role in the formation of cave systems. Earth Sci Rev 131:116–148. https://doi.org/10.1016/j.earscirev.2014.01.006
Dumurdžanov N, Serafimovski T, Burchfiel BC (2005) Cenozoic tectonics of Macedonia and its relation to the South Balkan extensional regime. Geol Soc Am: Geosphere 1(1):1–22. https://doi.org/10.1130/GES00006.1
Erőss A, Mádl-Szőnyi J, Surbeck H, Horváth Á, Goldscheider N, Csoma AÉ (2012) Radionuclides as natural tracers for the characterization of fluids in regional discharge areas, Buda thermal karst, Hungary. J Hydrol 426–427:124–137. https://doi.org/10.1016/j.jhydrol.2012.01.031
Erőss A, Csondor K, Czuppon G, Dezső J, Müller I (2020) Groundwater flow system understanding of the lukewarm springs in Kistapolca (South Hungary) and its relevance to hypogene cave formation. Environ Earth Sci 79:132. https://doi.org/10.1007/s12665-020-8870-3
Fontes J-C, Garnier JM (1979) Determination of the initial activity of the total dissolved carbon: a review of existing models and a new approach. Water Resour Res 12:399–413. https://doi.org/10.1029/WR015i002p00399
Ford D, Williams P (2007) Karst hydrogeology and geomorphology, 2nd edn. Wiley, Chichester, UK
Fournier R (1977) Chemical geothermometers and mixing models for geothermal systems. Geothermics 5:41–50. https://doi.org/10.1016/0375-6505(77)90007-4
Fournier R, Potter R (1979) Magnesium correction to the Na-K-Ca chemical geothermometer. Geochim Cosmochim Acta 43:1543–1550. https://doi.org/10.1016/0016-7037(79)90147-9
Fournier R, Truesdell A (1973) An empirical Na-K-Ca geothermometer for natural water. Geochim Cosmochim Acta 37:1255–1275. https://doi.org/10.1016/0016-7037(73)90060-4
Galdenzi S, Cocchioni M, Morichetti L, Amici V, Scuri S (2008) Sulfidic ground-water chemistry in the Frasassi caves, Italy. J Cave Karst Stud 70(2):94–107
Gary MO, Sharp JM (2006) Volcanogenic karstification of Sistema Zacatón, Mexico. In: Harmon RS, Wicks CW (eds) Perspectives on karst meomorphology, hydrology and geochemistry. GSA Special Paper 404, GSA, Boulder, CO, pp 79–89
Giggenbach W (1988) Geothermal solute equilibria. Derivation of Na-K-Mg-Ca geoindicators. Geochim Cosmochim Acta 52:2749–2765. https://doi.org/10.1016/0016-7037(88)90143-3
Goldscheider N, Mádl-Szőnyi J, Erőss A, Schill E (2010) Review: Thermal water resources in carbonate rock aquifers. Hydrogeol J 18(6):1303–1318. https://doi.org/10.1007/s10040-010-0611-3
Gonfiantini R, Zuppi GM (2003) Carbon exchange rate of DIC in karst groundwater. Chem Geol 197:319–336. https://doi.org/10.1016/S0009-2541(02)00402-3
Gunn J, Bottrell SH, Lowe DJ, Worthington SRH (2006) Deep groundwater flow and geochemical processes in limestone aquifers: evidence from thermal waters in Derbyshire, England, UK. Hydrogeol J 14:868–881. https://doi.org/10.1007/s10040-006-0022-7
Han LF, Plummer LN (2016) A review of single-sample-based models and other approaches for radiocarbon dating of dissolved inorganic carbon in groundwater. Earth Sci Rev 152:119–142. https://doi.org/10.1016/j.earscirev.2015.11.004
Hertelendi E, Veres M, Futó I, Svingor É, Mikó L, Lénárt L, Deák J, Süveges M (1995) Radiocarbon concentration and origin of thermal karst waters in the region of the Bükk Mountains, northeastern Hungary. Radiocarbon 37(2):543–550. https://doi.org/10.1017/S0033822200031039
Hua Q, Barbetti M, Rakowski AZ (2013) Atmospheric radiocarbon for the period 1950–2010. Radiocarbon 55(4):2059–2072. https://doi.org/10.2458/azu_js_rc.v55i2.16177
Ingerson E, Pearson FJ (1964) Estimation of age and rate of motion of groundwater by the 14C-method. In: Miyake Y, Koyama T (eds) Recent researches in the field of hydrosphere. Atmosphere and Nuclear Geochemistry, Tokyo, pp 263–228
Jasechko S, Birks SJ, Gleeson T, Wada Y, Fawcett PJ, Sharp ZD, McDonnell JJ, Welker JM (2014) The pronounced seasonality of global groundwater recharge. Water Resour Res 50:8845–8867. https://doi.org/10.1002/2014WR015809
Klimchouk AB (2007) Hypogene speleogenesis: hydrogeological and morphogenetic perspective. Special paper 1, National Cave and Karst Research Institute, Carlsbad, NM, 106 pp
Klimchouk A (2017) Types and settings of hypogene karst. In: Klimchouk A, Palmer A, De Waele J, Auler A, Audra P (eds) Hypogene karst regions and caves of the world. Cave and Karst Systems of the World book series, Springer, Cham, Switzerland, pp 1–39, https://doi.org/10.1007/978-3-319-53348-3_1
Klimchouk A, Palmer AN, De Waele J, Auler AS, Audra P (2017) Hypogene karst regions and caves of the world. Cave and Karst Systems of the World book series, Springer, Cham, Switzerland. https://doi.org/10.1007/978-3-319-53348-3
Kotevski G (1987) Hidrogeologija na mineralnite, termalnite i termomineralnite vodi na teritorijata na Socijalistička Republika Makedonija [Hydrogeology of the mineral, thermal and thermomineral waters on the territory of Socialist Republic of Macedonia]. Samupravna praktika, Skopje, Macedonia
Mádl-Szőnyi J, Tóth Á (2015) Basin-scale conceptual groundwater flow model for an unconfined and confined thick carbonate region. Hydrogeol J 23(7):1359–1380. https://doi.org/10.1007/s10040-015-1274-x
McKenzie W, Truesdell A (1977) Geothermal reservoir temperatures estimated from the oxygen isotope compositions of dissolved sulfate and water from hot springs and shallow drillholes. Geothermics 5:51–61. https://doi.org/10.1016/0375-6505(77)90008-6
Minissale A, Vaselli O, Tassi F, Magro G, Grechi GP (2002) Fluid mixing in carbonate aquifers near Rapolano (central Italy): chemical and isotopic constraints. Appl Geochem 17:1329–1342. https://doi.org/10.1016/S0883-2927(02)00023-9
Molnár M, Janovics R, Major I, Orsovszki J, Gönczi R, Veres M, Leonard AG, Castle SM, Lange TE, Wacker L, Hajdas I, Jull AJT (2013a) Status report of the new AMS 14C sample preparation lab of the Hertelendi Laboratory of Environmental Studies (Debrecen, Hungary). Radiocarbon 55(2–3):665–676. https://doi.org/10.2458/azu_js_rc.55.16394
Molnár M, Rinyu L, Veres M, Seiler M, Wacker L, Synal H-A (2013b) EnvironMICADAS: a mini 14C AMS with enhanced gas ion source Interface in the Hertelendi Laboratory of Environmental Studies (HEKAL), Hungary. Radiocarbon 55(2):338–344. https://doi.org/10.2458/azu_js_rc.55.16331
Mook WG (1980) Carbon-14 in hydrogeological studies. In: Fritz P, Fontes JC (eds) Handbook of environmental isotope geochemistry. Elsevier, Amsterdam, pp 49–74
Most T (2003) Geodynamic evolution of the Eastern Pelagonian Zone in northwestern Greece and the Republic of Macedonia: implications from U/Pb, Rb/Sr, K/Ar, 40Ar/39Ar geochronology and fission track thermochronology. PhD Thesis, University of Tübingen, Tübingen, Germany, 98 pp
Ozima M, Podosek FA (2002) Noble gas geochemistry, 2nd edn. Cambridge University Press, Cambridge, UK
Paceš T (1975) A systematic deviation from Na-K-ca geothermometer below 75°C and above 10−4 atm PCO2. Geochim Cosmochim Acta 39:541–544. https://doi.org/10.1016/0016-7037(75)90108-8
Palcsu L, Major Z, Köllő Z, Papp L (2010) Using an ultrapure 4He spike in tritium measurements of environmental water samples by the 3He-ingrowth method. Rapid Commun Mass Spectrom 24:698–704. https://doi.org/10.1002/rcm.4431
Palinkaš S, Hofstra A, Percival T, Borojević Šoštarić S, Palinkaš L, Bermanec V, Pecskay Z, Boev B (2018) Comparison of the Allchar Au-As-Sb-Tl deposit, Republic of Macedonia, with Carlin-type gold deposits. In: Muntean J (ed) diversity in Carlin-style gold deposits. Rev Econ Geol 20:335–363. https://doi.org/10.5382/rev.20.10
Palmer AN (2007) Cave geology. Cave Books, Dayton, OH
Palmer AN, Taylor PM, Terrell LA (2017) Hypogene karst springs along the northeastern border of the Appalachian Plateau, New York State. In: Klimchouk A, Palmer A, De Waele J, Auler A, Audra P (eds) Hypogene karst regions and caves of the world. Cave and karst systems of the world book series, Springer, Cham, Switzerland, pp 709–719. https://doi.org/10.1007/978-3-319-53348-3_47
Papp L, Palcsu L, Major Z, Rinyu L, Tóth I (2012) A mass spectrometric line for tritium analysis of water and noble gas measurements from different water amounts in the range of microlitres and millilitres. Isot Environ Health Stud 48(1):494–451. https://doi.org/10.1080/10256016.2012.679935
Parkhurst DL, Appelo CAJ (2013) Description of input and examples for PHREEQC version 3: a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. US Geological Survey Techniques and Methods, 6, A43, 497 pp. https://pubs.usgs.gov/tm/06/a43/. Accessed December 2020
Plan L, Tschegg C, De Waele J, Spötl C (2012) Corrosion morphology and cave wall alteration in an Alpine sulfuric acid cave (Kraushöhle, Austria). Geomorphology 169–170:45–54. https://doi.org/10.1016/j.geomorph.2012.04.006
Plummer LN, Prestemon EC, Parkhurst DL (1994) An interactive code (NETPATH) for modeling net geochemical reactions along a flow path, version 2.0. US Geol Surv Water Resour Invest Rep 94-4169. https://doi.org/10.3133/wri944169
Rinyu L, Molnár M, Major I, Nagy T, Veres M, Kimák Á, Wacker L, Synal H-A (2013) Optimization of sealed tube graphitization method for environmental 14C studies using MICADAS. Nucl Inst Methods Phys Res B 294:270–275. https://doi.org/10.1016/j.nimb.2012.08.042
Santaloia F, Zuffianò LE, Palladino G, Limoni PP, Liotta D, Minissale A, Brogi A, Polemio M (2016) Coastal thermal springs in a foreland setting: the Santa Cesarea Terme system (Italy). Geothermics 64:344–361. https://doi.org/10.1016/j.geothermics.2016.06.013
Schlosser P, Stute M, Dörr C, Sonntag C, Münnich KO (1988) Tritium/3He-dating of shallow groundwater. Earth Planet Sci Lett 89:353–362. https://doi.org/10.1016/0012-821X(88)90122-7
Spötl C, Desch A, Dublyansky Y, Plan L, Mangini A (2016) Hypogene speleogenesis in dolomite host rock by CO2-rich fluids, Kozak cave (southern Austria). Geomorphology 255:39–48. https://doi.org/10.1016/j.geomorph.2015.12.001
Sracek O, Geršl M, Faimon J, Bábek O (2019) The geochemistry and origin of fluids in the carbonate structure of the Hranice karst with the world’s deepest flooded cave of the Hranicka abyss, Czech Republic. Appl Geochem 100:203–212. https://doi.org/10.1016/j.apgeochem.2018.11.013
Tamers MA (1975) Validity of radiocarbon dates on groundwater. Geophys Surv 2:217–239. https://doi.org/10.1007/BF01447909
Temovski M (2016) Evolution of karst in the lower part of Crna Reka river basin. Springer Theses, Springer, Cham, Switzerland. https://doi.org/10.1007/978-3-319-24547-8
Temovski M (2017) Hypogene karst in Macedonia. In: Klimchouk A, Palmer A, De Waele J, Auler A, Audra P (eds) Hypogene karst regions and caves of the world. Cave and karst systems of the world book series, Springer, Cham, Switzerland, pp 241–256. https://doi.org/10.1007/978-3-319-53348-3_15
Temovski M, Palcsu L (2018) Geochemical characteristics of some thermal karst springs: insight into the hypogene karst systems in Mariovo, Macedonia. In: Milanović S, Stevanović Z (eds) Proceedings of the International Symposium KARST 2018 – Expect the Unexpected, Trebinje, Bosnia and Herzegovina, June 2018, pp 223–230
Temovski M, Audra P, Mihevc A, Spangenberg J, Polyak V, McIntosh W, Bigot J-Y (2013) Hypogenic origin of Provalata Cave, Republic of Macedonia: a distinct case of successive thermal carbonic and sulfuric acid speleogenesis. Int J Speleol 42(3):235–264. https://doi.org/10.5038/1827-806X.42.3.7
Temovski M, Futó I, Túri M, Palcsu L (2018) Sulfur and oxygen isotopes in the gypsum deposits of the Provalata sulfuric acid cave (Macedonia). Geomorphology 315:80–90. https://doi.org/10.1016/j.geomorph.2018.05.010
Vodila G, Palcsu L, Futó I, Zs S (2011) A 9-year record of stable isotope ratios of precipitation in eastern Hungary: implications on isotope hydrology and regional palaeoclimatology. J Hydrol 400:144–153. https://doi.org/10.1016/j.jhydrol.2011.01.030
Webb JA, Grimes KG, Lewis ID (2010) Volcanogenic origin of cenotes near Mt Gambier, southeastern Australia. Geomorphology 119(1):23–35. https://doi.org/10.1016/j.geomorph.2010.02.015
Wynn JG, Sumrall JB, Onac BP (2010) Sulfur isotopic composition and the source of dissolved sulfur species in thermo-mineral springs of the Cerna Valley, Romania. Chem Geol 271:31–43. https://doi.org/10.1016/j.chemgeo.2009.12.009
Acknowledgements
We would like to thank the members of SK Zlatovrv – Prilep for assistance during sampling. We are also thankful to the editor and the two anonymous reviewers for their useful comments and suggestions which improved the manuscript.
Funding
Open Access funding provided by ELKH Institute for Nuclear Research. This research was supported by the European Union and the State of Hungary, co-financed by the European Regional Development Fund in the project of GINOP-2.3.2-15-2016-00009 ‘ICER’
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 99 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Temovski, M., Túri, M., Futó, I. et al. Multi-method geochemical characterization of groundwater from a hypogene karst system. Hydrogeol J 29, 1129–1152 (2021). https://doi.org/10.1007/s10040-020-02293-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10040-020-02293-w