Abstract
Increasing atmospheric CO2 enhances plant biomass production and may thereby change nutrient concentrations in plant tissues. The objective of this study was to identify the effect of elevated atmospheric CO2 concentrations on nutrient concentrations of grassland biomass that have been grown for 16 years (1998–2013). The grassland biomass grown at the extensively managed Giessen FACE experiment, fumigated with ambient and elevated CO2 (aCO2; eCO2; +20%) was harvested twice annually. Concentrations of C, N, P, K, Ca, Mg, Mn, Fe, Cu and Zn were determined separately for grasses, forbs and legumes. Under eCO2, the concentration of N was reduced in grasses, Ca was reduced in grasses and forbs, P was reduced in grasses but increased in legumes, Mg concentration was reduced in grasses, forbs and legumes and K was reduced in grasses but increased in forbs. The nutrient yield (in g nutrient yield of an element per m−2) of most elements indicated negative yield responses at a zero biomass response to eCO2 for grasses. K and Zn nutrient yields responded positively to eCO2 in forbs and Mn and Fe responded positively in forbs and legumes. The results suggest that under eCO2 the nutrient concentrations were not diluted by the CO2 fertilization effect. Rather, altered plant nutrient acquisitions via changed physiological mechanisms prevail for increased C assimilation under eCO2. Furthermore, other factors such as water or nutrient availability affected plant nutrient concentrations under eCO2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Grasses revealed most reduced element concentrations due to eCO2.
-
In grasses, most elements indicated a negative nutrient yield under eCO2.
-
Site and climatic conditions affected CO2 effect on nutrients concentrations.
Introduction
Grassland ecosystems play a critical role in providing food and forage to a large number of the world’s human population and their livestock (White and others 2000). Elevated atmospheric CO2 (eCO2) is an important influencing factor on grassland vegetation often driving increased biomass production under eCO2 (Andresen and others 2018; Cantarel and others 2013; Feng and others 2015; Newton and others 2014).
Elevated CO2 can induce direct plant physiological reactions such as reduced stomatal conductance and elevated photosynthetic rates (Haworth and others 2016; Tausz-Posch and others 2014). Together, these physiological reactions affect the plant nutrient uptake by downregulating the passive inflow of nutrients to the root with the transpiration stream (Houshmandfar and others 2018) and possibly stimulate a more active uptake of certain elements required in photosynthesis. Shifts of photosynthetic activity under eCO2 can lead to limited electron transport, which can decrease plant nutrient requirements and uptake. However, concentrations of micronutrients such as Fe and Mn, which are involved in electron transport, have been found to increase under eCO2 (Natali and others 2009). On the other hand, plant uptake of nutrients like Ca and Mg is controlled by mass flow, which associated with the transpiration stream (Nord and Lynch 2009). Hence, decreased transpiration rates by eCO2 and therefore decreased mass flow may lead to decreased uptake of nutrients such as Ca and Mg in aboveground plant biomass (Houshmandfar and others 2018; Tausz-Posch and others 2014).
Ainsworth and others (2003) revealed increased C sink strengths due to elevated photosynthesis in grassland plants grown under eCO2. Moreover, plants grown under eCO2 increased carbohydrate contents, which led to the explanation of dilution of nutrient concentrations in plant tissues by increased C assimilation (Ainsworth and Long 2021; Loladze 2002). However, in a meta-analysis, Feng and others (2015) showed that the N nutrient yield was generally reduced under eCO2 in grassland, cropland and forest ecosystems, likely due to reduced N acquisition under eCO2 and not due to C dilution. Similar evidence was reported by Houshmandfar and others (2018) for nutrient uptakes Ca, K, N, S, Mg and Mn in wheat that were correlated with plant transpiration.
Besides the direct effects on plant productivity, biodiversity and physiological responses, eCO2 can also indirectly and directly affect soil nutrient cycles (Hawkesford and others 2014). Availability of nutrients is also controlled by soil properties, such as pH, which affects nutrient ion diffusion and adsorption (Marschner 2002). A slight decrease in soil pH can be expected under eCO2 conditions because of increased root exudation and respiration, changed cation–anion exchange balance or organic anion release (Hinsinger and others 2003; Natali and others 2009). Additionally, increasing inputs of carbonic acid from plant roots and microbial respiration, due to eCO2, may increase soil acidity (Natali and others 2009). For slightly acidic soils, such a pH decrease could directly affect the availability of soil nutrients. The effect would be a decrease in the availability of macronutrients such as N, P, K, Ca, S and Mg and an increase in the availability of micronutrients such as Fe, Mn, Zn and Cu (Marschner 2002), which might in turn affect the nutritional status of plants (Chapin 1980).
One of the most important plant nutrients is N, and a controlling factor for soil N availability is the process of mineralization of soil organic matter. Net N mineralization varied strongly in response to eCO2 in grasslands (Hovenden and others 2017; Reich and others 2018), whereas gross N mineralization rates were often not responding to eCO2 (Müller and others 2009; Rütting and Andresen 2015; Rütting and Hovenden 2020). Furthermore, the presence of legumes in ecosystems adds to the complexity as these are often associated with N2 fixing microorganisms (Gamper and others 2004; Lüscher and Nösberger 1997). Legumes are found to benefit even more under eCO2 as the N2 fixation increases due to increasing nodule size, number of nodules per plant and a higher nitrogenase activity (Rogers and others 2009). The N supply via symbiotic N fixation can, through years, sustain an otherwise decreasing N availability for all plant species (Liang and others 2016; Rütting 2017). In ecosystems without N2 fixing organisms, a situation with a progressive nitrogen limitation (PNL) has been predicted but rarely observed. For PNL, a decline in plant available N can restrain the biomass from responding positively to eCO2 (Newton and others 2010). Hence, changes in soil N availability for plant uptake are difficult to predict under eCO2.
Across many short-term FACE experiments, decreases in plant nutrient concentrations have been observed, usually with small but significant effects (Myers and others 2014). These changes in the nutrient concentrations suggest an adjustable plant nutrient stoichiometry; however, an increased C-to-N ratio under eCO2 is often observed (Dijkstra and others 2012; Feng and others 2015; Sistla and Schimel 2012). Also, in a meta-analysis of 1418 studies, Yuan and Chen (2015) found that N-to-P ratios generally decreased under eCO2. However, observations suggest high variability in responses. Lower concentrations of Zn and Fe in response to eCO2 were also observed in wheat, rice, beans and soybeans (Myers and others 2014; Smith and Myers 2018), indicating that the nutritive value of crops decline with negative impacts on human and livestock nutrition (Augustine and others 2018; Dumont and others 2015; Myers and others 2014; Seibert and others 2021; Smith and Myers 2018; Yuan and Chen 2015).
Long-term continuous observations of nutrient concentrations in eCO2 experiments over more than 10 years are rare. Investigations on the species rich, extensively managed, temperate grassland at the long-term Giessen Free-Air Carbon dioxide Enrichment experiment (GiFACE) in Germany (Jäger and others 2003; Müller and others 2019) revealed eCO2 effects on aboveground biomass yield and its forage quality (Andresen and others 2018; Kammann and others 2005; Obermeier and others 2017; Seibert and others 2021; Yuan and others 2018). During the study period at the GiFACE experiment since 1998 (Jäger and others 2003), the biomass of grasses decreased, while forbs (including legumes) increased in proportion (Andresen and others 2018; Seibert and others 2021). Furthermore, the total biomass production increased (+15%) under eCO2 (Andresen and others 2018) and the forage quality declined, especially in forbs (Seibert and others 2021).
To reveal which of the macro- and micronutrients were affected by eCO2, we addressed the following hypotheses:
-
1.
We expected differences in N concentration and its CO2 response among the functional groups because of the different CO2 responses of plant N uptake in forbs and grasses and the N2 fixation mechanism in symbionts with legumes.
-
2.
We expected decreased leaf concentrations of Ca, K, N, S and Mg under eCO2, due to downregulated transpiration and therefore reduced passive inflow of nutrients and lower availability of these nutrients due to a slight soil acidification.
-
3.
We expected increased nutrient concentrations in response to eCO2 for Fe and Mn due to stimulated active uptake to meet increased photosynthesis under eCO2 and because a slight acidification under eCO2 increases the availability of these elements.
Materials and Methods
Field Site
The GiFACE site is a 1.5 ha large research site in the German state Hessen, near Giessen (50°32’N 8°41’E) at 172 m.a.s.l. The soil is a Fluvic Gleysol in a floodplain. Since more than 100 years, the area has been managed as permanent grassland (seminatural). Until 1995, the site was fertilized with 50–80 kg N ha−1 y−1. Since 1993, the commercial fertilizers ‘Thomas-Kali’ and ‘Kalk-Ammon-salpeter’ were applied once a year in April at the beginning of the growing season. Since 1996 a fertilization level of 40 kg N ha−1 y−1 was applied, which is equivalent to the following nutrient amounts: nitrogen: 4 g N m−2 y−1, phosphorus: 6 g P m−2 y−1, potassium: 9 g K m−2 y−1, magnesium: 1.8 g Mg m−2 y−1 and calcium: 21.3 g Ca m−2 y−1 (Table S1, Andresen and others 2018). The mean content of the soil organic matter at a depth of 7.5 cm is 2952 (± 255) C g m−2 for the aCO2 plots and 2924 (± 146) C g m−2 for the eCO2 plots (Keidel and others 2018).
The vegetation is an ‘Arrhenatheretum elatioris—Filipendula ulmaria sub-community’ (Grüters and others 2006) according to the Braun-Blanquet classification. At the start of the study, the dominating grasses in terms of biomass were: Arrhenatherum elatius (L) P. Beauv. ex J. Presl & C. Presl., Holcus lanatus L, Trisetum flavescens (L) P. Beauv., Poa pratensis L., Anthoxanthum odoratum L. and Poa trivialis L., the dominant forbs were Galium album Forssk., Geranium pratense L., Plantago lanceolata L., Filipendula ulmaria (L.) Maxim. and Sanguisorba officinalis L.. The legume species Lathyrus pratensis L., Trifolium pratense L. and Trifolium repens L. were present in small proportions (< 1–2% by mass) when this study was initiated (Grüters and others 2006).
Elevated CO2 Treatments
Three circular plots (8 m inner diameter rings) were subjected to CO2 enriched air (eCO2; + 20% above ambient conditions), while three rings were subjected to ambient CO2 concentrations (aCO2), arranged in a randomized block design (3 blocks). CO2 fumigation started in May 1998 and continued all-year round, from 2 h after astronomical sunrise until 2 h before astronomical sun set (Jäger and others 2003).
Local Weather and Climatic Conditions
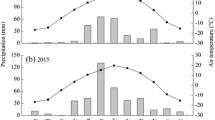
Volumetric soil water content was measured daily since spring 1997 by 4 permanent TDR sensors (Imko, Germany, type P2G) in 0–15 cm depth in each ring. Soil temperature was recorded every 30 min since March 1998 at 10 cm depth with 3 probes in each ring (Pt-100 sensors, Imko, Germany). The precipitation sum was measured at the site in 30-min intervals; the mean annual precipitation was 556 ± 27 mm (average ± standard deviation (SD)); 18 year average from 1995 December to 2013 November) with the seasonal averages during winter (December to February) of 108 ± 33 mm; spring (March to May) 130 ± 48 mm; summer (June to August) 172 ± 49 mm; and autumn (September to November): 142 ± 55 mm. The precipitation data were used to calculate the 3 months standardized precipitation index (SPI), which is a drought index based only on precipitation (WMO 2012). Two meter aboveground temperature was recorded continuously since 1995 at two stations within the field site. The mean annual temperature was 9.4 ± 6.5 °C with the seasonal averages: winter 1.44 ± 1.67 °C; spring 9.24 ± 1.02 °C; summer 17.37 ± 0.78 °C; and autumn 9.50 ± 1.01 °C. The aboveground temperature data were used to calculate the 3 months standardized temperature index (STI), representing the probability of temperature value occurrences when compared with temperature average of a longer period (Fasel 2015). For details and further data presentations, see Andresen and others (2018) and Obermeier and others (2017), Seibert and others (2021) and Yuan and others (2018).
Biannual Harvest
Since 1993, harvests were conducted within 25 subplots with an area of 0.36 m2 per subplot per ring (Andresen and others 2018). All vegetation was cut with garden scissors at 3–5 cm above soil surface on two occasions each year: harvest 1 at the end of May and harvest 2 at the beginning of September. The fresh biomass was stored under cool conditions and sorted by hand into three functional groups: grasses, forbs and legumes and was then oven-dried at 105 °C. The dried material was ground in a mill (SM 300, Retsch, Germany). From each subplot a representative sample (relative to the biomass yield in each subplot) was mixed to one composite sample from the ring for further chemical analysis.
Plant Leaf Nutrient Analysis
The element concentration of dried ground plant material was analyzed by standard methods. The samples for the years 1993–2013 were analyzed for total N and C with a Vario Max CNS (Elementar). Other elements were analyzed in two large batches assembled through the years. Plant samples from the years 1993 to 2006 were analyzed at the Agri-Food and Biosciences Institute, Belfast, where they were digested by microwave using nitric acid (0.25 g plant material was added 5 ml concentrated nitric acid) in 45 ml deionized water to give a final volume of 50 ml. The elements were then determined using an ICP-MS (inductively coupled plasma mass spectrometer; Varian Liberty Series II) for analysis of P, K, Ca, Mg, Mn, Fe, Cu, Zn and S. Plant samples from the years 2007 to 2013 were analyzed at Geisenheim University laboratories in the department of soil science and plant nutrition, where they were digested at 400 °C by a Kjeldahl mixture consisting of: hydrochloric acid, peroxide, lithium sulfate, selene (0.6 g plant material + 10 ml) for analysis of P, K, Ca, Mg, Mn, Fe, Cu and Zn with an ICP-OES (Arcos) and of N at a FIA (Foss). A small-scale comparison between laboratories was made with samples that were initially analyzed in Belfast and then re-analyzed in Geisenheim (7 years later) which confirmed that there was no baseline shift for all elements except for Fe and Cu. S was not analyzed in Geisenheim and thus determined only until 2006. However, since all samples from any harvest time were always consistently analyzed within the same laboratory, this did not have consequences for determining the CO2 treatment effect. Legume biomasses were only analyzed when sufficient plant material was available.
Data Analysis
Plant leaf concentrations of the elements N, P, K, Ca, Mg, Fe, Zn, Mn and Cu in each of the plant functional types grasses, forbs and legumes were used for data analysis separately for harvest 1 (H1) and harvest 2 (H2).
The plant nutrient yield of a nutrient element (Ni) was calculated per functional group and harvest according to the following equation (Tausz-Posch and others 2014).
The annual nutrient yield for an element was the summed yield of Ni from the two harvests (H1 and H2) and all functional groups.
The eCO2 treatment effect on plant growth and nutrient yield was quantified by determining the effect size, respectively. Effect size expresses the relative treatment effect in percentage (Leuzinger and others 2011) and was calculated as follows for the biomass X (each functional group) or the nutrient yield of element Ni (Ni represents the average of the three treatment rings for each functional group):
To compare the functional groups of all six FACE rings, separated in eCO2 and aCO2, as well as in H1 and H2, a principal component analysis (PCA) biplot of nutrient concentrations was used. Cross product matrix contains correlation coefficients among nutrient concentrations and 7 axes were interpreted (McCune and Grace 2002). Before analysis, the data were subjected to square root transformation (van der Maarel 1979). The PCA was performed using PC-Ord 6 (McCune and Mefford 2011). To analyze the coordinated effect of eCO2 on plant nutrient concentrations, a one-way permutational multivariate analysis of variance (PerMANOVA) with Sorensen distance measure was carried out for each functional group and harvest (Anderson and Walsh 2013; McCune and Grace 2002).
To test the significance of the differences of all nutrient concentrations and yields between eCO2 and aCO2, a linear mixed model analysis with repeated measures was used for the dataset 1998–2013. As covariance type, first-order autoregressive process [AR(1)] was used. All mixed model analyses were carried out in SPSS version 23.0 (IBM Corp., Armonk, NY, USA).
The model analysis was started with the full-factorial model with the factors CO2, block, year and the co-variables soil moisture, STI and SPI, including the interactions CO2 × block, CO2 × year, CO2 × soil moisture, CO2 × STI, CO2 × SPI, block × soil moisture, block × STI, block × SPI, year × soil moisture, year × STI and year × SPI. Soil moisture, STI and SPI data were aggregated to averages for the respective 3 months preceding the harvest (March–May for H1 and June to August for H2). The model was reduced for nonsignificant interactions (p ≥ 0.1) and evaluated for their goodness of fit based on the Akaike information criterion (Andresen and others 2018; Bandoly and others 2016; Safari and others 2016). Effects with p values ≤ 0.05 were considered significant, ≤ 0.01 very significant and ≤ 0.001 highly significant.
Nutrient Uptake Response
Linear regression was made for the CO2 responses measured as effect size (Eq. 2) of nutrient yield (Yi) versus the effect size of biomass (x):
The intercept with the Y-axis (Y0) at xi = 0 is the percent reduction or increase in nutrient yield at zero biomass response. If this intercept is a negative number for the theoretical point of no response in biomass, it means the nutrient yield under eCO2 is smaller than under aCO2. We judged significant negative (or positive) Y0 by its 95% confidence interval (CIup and CIlow) including ‘0’ or not. We compared the Y0 across element and functional types as expression of loss in nutrient yield under eCO2. SMATR (a freeware program for standardized major axis tests and routines; Falster and others 2006; Warton and others 2006) was used for bivariate linear regression, using standardized major axis regression and testing for ‘common slopes’ (p(test) < 0.05) of the regression lines being compared. For each element, we compared in Eq. (3) the plant-type grasses, forbs, legumes pairwise. A difference of slopes indicates that one functional plant type has a better nutrient uptake under eCO2 than the other type.
Results
Concentration of Nutrients Aboveground in Plant Functional Types
Elevated CO2 decreased the nitrogen (N) concentration in grasses only for H2 and not in forbs and legumes (p = 0.001; Figure 1a and b; Table 1; Table S2; linear mixed model).
Concentrations of N (a, b, c), P (d, e, f), Ca (g, h, i), Mg (j, k, l) and K (m, n, o) in % of grasses, forbs and legumes, separated in harvest 1 (H1; circles) and harvest 2 (H2, triangles). Error bars indicate standard deviation. The results of the mixed model analysis are indicated for treatment (CO2), block (Bl.), time (Y) and soil moisture (SWC). The significance levels were reported as significant p ≤ 0.05 [*], much significant p ≤ 0.01 [**] and highly significant p ≤ 0.001 [***]. Highly significant results in bold
Phosphorus (P) had both negative and positive eCO2 effects seen as declined concentrations in grasses (H1, p = 0.011; H2, p = 0.024; Figure 1d; Table 1; Table S2) and increased concentrations in legumes (H2, p = 0.045; Figure 1f; Table 1; Table S4). There was also a significant CO2 × soil moisture interaction for P in grasses H1 (Table S2; Table S3).
CO2 had a negative effect for calcium (Ca) in grasses, with decreased concentrations under eCO2 for both harvests, (both p ≤ 0.001; Table 1; Figure 1g; Table S2). Forbs also showed lower Ca concentrations under eCO2 (p = 0.030; Table 1; Figure 1h; Table S3).
The magnesium (Mg) concentrations decreased significantly in all functional groups and both harvests under eCO2 (Table 1; Table S2, S3 and S4). Mg concentrations were smaller under eCO2 for both harvests in grasses (Figure 1j), except in 2013 for H1 (H1, p = 0.008; H2, p = 0.004; Figure 1j; Table 1; Table S2). Forbs had a negative eCO2 effect for both harvests (H1, p = 0.005; H2, p = 0.043; Table 1; Table S3), except in 2000 for H1 and in 2005, 2008, 2010 and 2013 for H2 (Figure 1k). In legumes, a smaller Mg concentration under eCO2 occurred for the whole time series for H1 (p = 0.009; Figure 1l; Table 1; Table S4) and with only one exception in 2000 for H2 (p = 0.030; Figure 1l; Table 1; Table S4).
Potassium (K) concentrations in grasses showed negative eCO2 effects for both harvests, with decreasing K concentrations, except in 2000 for H2 (H1, p = 0.041; H2, p = 0.024; Figure 1m; Table 1; Table S2). Forbs had a positive CO2 effect for H2, with higher K concentrations under eCO2, except in 2010 (p = 0,036; Figure 1n; Table 1; Table S3).
Concentrations of Zn, Fe, Cu and Mn indicated no significant CO2 effects in any of the functional groups (Table 1). Significant effects of site conditions (‘block’), year and interactions in the linear mixed model are presented in detail in the supplementary tables (Table S2; Table S3; Table S4).
A joint analysis of the three functional groups (grasses, forbs and legumes) revealed a clear separation among all nutrient concentrations under ambient and elevated CO2 by the un-rotated s-mode principal components analysis (PCA), which also revealed a clear separation between H1 (spring) and H2 (summer) (Figure S1). However, a clear effect in the functional groups as a response to CO2 treatment was not found and this was confirmed by the PerMANOVA analysis (Table S5).
Plant Nutrient Yield Responses
The response to eCO2 measured as effect size (Eq. 3) of the nutrient yields varied as both positive and negative for all elements and functional groups across all years (Eq. 2, data not shown), and this inherently followed the temporal trends from the biomass response (Andresen and others 2018). The nutrient yield response relative to the biomass response correlated positively between all functional types (Table S6). For all elements, these slopes were not different for grasses and forbs except for Fe, where grasses had the steepest slope. Furthermore, for all elements the slopes were not different for grasses and legumes, except for Fe and Mn, where again grasses had a steeper slope. Forbs and legumes had common slopes for all elements, except Zn, where the slope for legumes was steepest (Table S6).
A relative reduction of nutrient yield under eCO2 compared to aCO2 in zero biomass response was found for grasses, as a negative Y-axis intercept Y0 (Eq. 3) occurred for all elements, significantly for K, Ca, Mg, Fe, Mn, Cu and S (95% confidence interval excluding the zero point; Figure 2a; Table S6). Forbs, however, had mainly negative Y0 for N, P, Ca, Mg and S but had contrastingly significant positive Y0 for Mn and Zn (Figure 2b). For legumes, the Y0 was negative only for Mg and positive for Mn, (Figure 2c). The largest reduction of Fe and Mn nutrient yields (− 63% and − 20%, respectively; Y0 in Table S6) was found in grasses, although they had smaller yield reductions of other nutrients (between 1 and 10%). Contrastingly, forbs and legumes showed the largest yield increase of Mn of 8% and 10% (Figure 2b and c; Table S6). The mean N yield of grasses and forbs were about 3% reduced, while the mean N yield of legumes was 5% reduced (for example, Y0 = − 3.29%, − 2.66% and − 5.44%, respectively; Table S6).
Response to CO2 for the macronutrient elements K, N, Mg, P, Ca, S and the micronutrient elements Cu, Mn, Fe and Zn, seen as the effect size of plant nutrient yield (% reduction or increase from eCO2) at Y0 which is the extrapolated point of zero biomass response to eCO2, jointly for harvest 1 and 2. The intercept Y0 was found using standardized major axis test considering variance at both axes (SMATR) on the response function of the nutrient (i) yield response (Y) relative to the biomass response (x) (Eq. 3) for each of the three functional groups grasses (a), forbs (b) and legumes (c) and is presented in Table S6. Y0 is the intercept with Y-axis at X = 0 and is the percentage reduction of (or increase in) nutrient yield for zero biomass response. The bar length represent the 95% confidence interval of the intercept Y0 and the line in the middle of each bar represents the mean value. Asterisks (*) emphasize significance where all both upper and lower confidence interval excludes (or includes) point zero
Discussion
In this study, we found that eCO2 reduced plant nutrient concentrations in grasses (N, P, Ca, Mg, K; Table 1; Figure 1), although eCO2 had reducing effects only for Ca and Mg concentrations in forbs and legumes, but showed increasing effects on K and P concentrations in forbs and in legumes, respectively (Table 1; Figure 1). The nutrient yield response Y0 (Figure 2 and Table S6) also pointed toward reduced nutrient yields in grasses, while in forbs and legumes they increased and reduced. This partly confirmed our hypothesis 1 of differences among the functional groups regarding N, but the increase in concentration and yields contradicted hypothesis 2 which suggested a decrease. Compared to forbs and legumes, the concentration reductions in grasses were more intense for H2 than for H1 (Table 1). This reduction occurred especially in H2 at the end of the regrowth phases, when the availability of spring applied fertilizer nutrients was low and dry soil conditions caused reduced transpiration rates. The GiFACE management protocol requires only one fertilization during the first growing period and requires the removal of the biomass upon harvest; therefore, plant nutrients were relatively more limited at H2 than at H1. Provided that the spring fertilization is enhancing nutrient availability for all species, the physiological differences of the functional groups for nutrient acquisition may become more important to explain the observed differences. As stated in hypothesis 3, increased active uptake of Fe and Mn is expected as consequence of upregulated photosynthesis under eCO2. Contrastingly to this hypothesis, grasses had a reduced Fe and Mn yield, suggesting downregulated photosynthesis, however, forbs and legumes were in line with the upregulation hypothesis.
Three hypothetical physiological mechanisms may decrease plant nutrient concentrations under eCO2: (i) dilution in the plant tissue of the acquired nutrient amount by an increased C assimilation (Ainsworth and Long 2021; Loladze 2002), (ii) decreased water inflow due to improved water use efficiency, carrying fewer nutrients (Houshmandfar and others 2018; Tausz-Posch and others 2014) or (iii) decreased water mass flow caused by downregulation of photosynthesis because of low C sink strength (Ainsworth and others 2003) and therefore reduced stomatal conductance.
The meta-analysis across FACE sites by Feng and others (2015) suggested that the dilution (i. above) in plant tissue cannot fully explain reduced N yields, rather a reduced uptake of N is realistic (ii. and iii. above), which was the case in our study, as indicated by the negative Y-axis intercept Y0 for zero biomass response to eCO2 (Figure 2; Table S6). This negative Y0 implies that the nutrient yield was still smaller under eCO2 compared to aCO2, when the biomass was non-responding to eCO2. Between elements and functional groups the Y0 indicated differences in the eCO2 response in nutrient yield versus response in biomass in the GiFACE (Figure 2). For K, Mg, Ca, S, Cu, Mn, Fe in grasses, P, Ca, S in forbs and Mg in legumes the significantly negative Y0 indicated that eCO2 had a negative effect on nutrient yield, independent of the effect on biomass. Hence, these plant nutrient yields are in effect not responding proportionally to productivity stimulation with increased C assimilation, which indicates that nutrient dilution does not explain reduced nutrient concentrations.
The mechanisms behind the reduced nutrient yield under eCO2 can be related to plant physiological constraints, such as root cell absorption sites (Welch and Shuman 1995), or a high assimilation efficiency and therefore a reduced nutrient inflow via reduced transpiration under eCO2 (Haworth and others 2016). Furthermore, a downregulation of photosynthesis and low C sink strength (Ainsworth and others 2003) can reduce nutrient yields. Soil properties like soil pH, buffer capacity, soil moisture and soil structure affect the solubility and sorption of most nutrient elements in the soil (Hawkesford and others 2014). Investigations by Brenzinger and others (2017) at the GiFACE site indicated only marginally and nonsignificant differences in soil characteristics (that is, pH, soil moisture, C content, N content) between eCO2 and aCO2. Results by Moser and others (2018) in the GiFACE indicated increased N mineralization rates under eCO2 that contributed to increased N2O emissions and caused also changed nutrient availability and competition between plants and microbes. Reduced pH, due to eCO2, is a widespread effect in different studies (Hinsinger and others 2003; Natali and others 2009) leading to an increasing (for example, Fe, Mn, Zn and Cu) or decreasing (for example, N, P, K, Ca, S and Mg) availability of nutrients for plants. Even though no pH shift in bulk soil was observed in the GiFACE (Brenzinger and others 2017), this pH controlled nutrient availability seems to be partly reflected in the rhizosphere by the observed forb (and legume) nutrient yield responses, for example, more Fe, Mn and Zn yield and less N, P, Ca, Mg yield (Figure 2) and therefore at least partly confirmed our second and third hypothesis. However, the grass nutrient yield response does not support these hypotheses. In that case the limited C sink strength compared to the one of forbs and legumes might cause a downregulation of photosynthesis and therefore stomata aperture in grasses causing reduced transpiration and reduced passive nutrient uptake by reduced water mass flow. An analysis of wheat under eCO2 showed an increase in the ratio of nutrient uptake per unit of transpired water for Ca, Mg and Mn, supporting a compensatory mechanism, albeit insufficient to prevent a decline in nutrient contents (Houshmandfar and others 2018).
The CO2 effect on nutrient concentrations was dependent on site and seasonal climatic conditions which control transpiration and photosynthetic assimilation, as was evident from significant interactions of the tested factors (Table S2; Table S3; Table S4). This was in line with previous studies at the GiFACE, where the CO2 fertilization effect on biomass yield (Andresen and others 2018), as well as the CO2 effect on forage quality (Seibert and others 2021) showed high dependency on abiotic conditions. The highest CO2 fertilization effects occurred under warm and humid climatic conditions during the growth periods, which were similar to the long-term average conditions of the respective seasons. Climatic conditions below or above the long-term average conditions lead to a reduction and suppression of the CO2 fertilization effect (Obermeier and others 2017; Yuan and others 2018). For example, during extreme climatic events like heat and drought, transpiration reduced via stomata aperture and therefore the CO2 assimilation and fertilization effect was reduced. During cold and wet seasons, plant transpiration and therefore nutrient uptake via mass flow were reduced by low vapor pressure deficit.
In the current analysis, P and Ca in grasses H1 showed significant negative CO2 effects, but the significant site conditions (denoted as block and soil moisture effect) and interactions (that is, CO2 × soil moisture in P and CO2 × block in Ca; Table S2) put the CO2 effect into perspective. These interactions indicated the dependence of the CO2 fertilization effect on soil moisture. The significant block × soil moisture interaction in Mg and K for grasses H1 (Table S2), reflects the moisture gradient at the GiFACE site, which led to the block partitioning of the FACE rings (Jäger and others 2003). Nevertheless, this interaction did not affect the negative eCO2 effect for Mg concentration, seen by the CO2 effect (p = 0.008; Table S2; Figure 1j). However, K concentrations indicated further significant interactions, like for grasses H2 (Table S2), which again relativized the CO2 effect. The same pattern was seen for Mg (H1 and H2) and K (H1) in forbs and K (H1) in legumes. This dependence of the CO2 fertilization effect on site and climatic conditions and the occurrence of extreme climatic events (denoted as year) with significant factor interactions were also confirmed by the forage quality analysis of Seibert and others (2021), seen in the quality parameter ash.
Conclusion
Our results revealed differences in plant nutrient concentrations and nutrient yield responses in an extensively managed grassland throughout 16 years of CO2 enrichment. In particular, plant functional types of grasses, forbs and legumes differed in their nutrient specific responses to eCO2. Most element concentrations were reduced under eCO2, especially for grasses, whereas single element concentrations in forbs and legumes showed a positive eCO2 effect of nutrient yields. This supports the hypothesis that under eCO2 the plant nutrient concentration was not generally diluted by the CO2 fertilization effect through increased C assimilation, but that it was caused by altered plant nutrient acquisition. For grasses, the general reduced plant nutrient yield was likely due to physiological mechanisms, such as reduced water uptake because of a downregulation of photosynthesis due to C sink limitation and therefore reduced photosynthesis and transpiration via reduced stomata aperture. Furthermore, we suggest that an additional soil acidification in the rhizosphere under eCO2 might slightly decrease the availability of N, P, K, Ca, S and Mg and slightly increase the availability of Fe, Mn, Zn and Cu to plants.
We conclude that the nutritive value of extensively managed temperate grassland may be reduced in response to eCO2. In particular, Ca, Mg, N, P and K indicated a negative response to eCO2 compared to the remaining investigated elements. Particularly grasses are more affected by this process than forbs and legumes because of different C sink strength. However, it appears that the CO2 effect on nutrient concentrations is most pronounced under average site and climatic conditions, which do not restrict the CO2 fertilization effect on plant biomass, while it is reduced during extreme weather events. As soon as the CO2 fertilization effect is limited by other factors like water or nutrient availability, interactions dominate the nutrient concentrations under eCO2. These observed changes in nutrient contents and yields of grassland biomass may have serious impact on ecosystem services, for example forage production and grassland management, and adjustment of fertilization regimes might be needed.
References
Ainsworth EA, Long SP. 2021. 30 years of free-air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Global Change Biology 27:27–49.
Ainsworth EA, Davey PA, Hymus GJ, Osborne CP, Rogers A, Blum H, Nosberger J, Long SP. 2003. Is stimulation of leaf photosynthesis by elevated carbon dioxide concentration maintained in the long term? A test with Lolium perenne grown for 10years at two nitrogen fertilization levels under free air CO2 enrichment (FACE). Plant, Cell & Environment 26:705–714.
Anderson MJ, Walsh CI. 2013. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecological Monographs 83:557–574.
Andresen LC, Yuan N, Seibert R, Moser G, Kammann CI, Luterbacher J, Erbs M, Müller C. 2018. Biomass responses in a temperate European grassland through 17 years of elevated CO2. Global Change Biology 24:3875–3885.
Augustine DJ, Blumenthal DM, Springer TL, LeCain DR, Gunter SA, Derner JD. 2018. Elevated CO2 induces substantial and persistent declines in forage quality irrespective of warming in mixedgrass prairie. Ecological Applications 28:721–735.
Bandoly M, Grichnik R, Hilker M, Steppuhn A. 2016. Priming of anti-herbivore defence in Nicotiana attenuata by insect oviposition: herbivore-specific effects. Plant, Cell and Environment 39:848–859.
Brenzinger K, Kujala K, Horn MA, Moser G, Guillet C, Kammann C, Müller C, Braker G. 2017. Soil conditions rather than long-term exposure to elevated CO2 affect soil microbial communities associated with N-cycling. Frontiers in Microbiology 8:1976. https://doi.org/10.3389/fmicb.2017.01976.
Cantarel AAM, Bloor JMG, Soussana J-F. 2013. Four years of simulated climate change reduces aboveground productivity and alters functional diversity in a grassland ecosystem. Journal of Vegetation Science 24:113–126.
Chapin FS III. 1980. The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:33–260.
Dijkstra FA, Pendall E, Morgan JA, Blumenthal DM, Carrillo Y, DR, Lecain, RF, Follett, Williams DG. 2012. Climate change alters stoichiometry of phosphorus and nitrogen in a semiarid grassland. New Phytologist 196:807–815.
Dumont B, Andueza D, Niderkorn V, Lüscher A, Porqueddu C, Picon-Cochard C. 2015. A meta-analysis of climate change effects on forage quality in grasslands: specificities of mountain and Mediterranean areas. Grass and Forage Science 70:239–254.
Falster, D., Warton, D., Wright, I. (2006). Retrieved from: http://bio.mq.edu.au/research/groups/ecology/SMATR/index.html
Fasel, M. (2015), Retrieved from: https://cran.r-project.org/web/packages/STI/STI.pdf
Feng Z, Rütting T, Pleijel H, Wallin G, Reich PB, Kammann CI, Newton PCD, Kobayashi K, Luo Y, Uddling J. 2015. Constraints to nitrogen acquisition of terrestrial plants under elevated CO2. Global Change Biology 21:3152–3168.
Gamper H, Peter M, Jansa J, Lüscher A, Hartwig UA, Leuchtmann A. 2004. Arbuscular mycorrhizal fungi benefit from 7 years of free air CO2 enrichment in well-fertilized grass and legume monocultures. Global Change Biology 10:189–199.
Grüters U, Janze S, Kammann C, Jäger H-J. 2006. Plant functional types and elevated CO2: A method of scanning for causes of community alteration. Journal of Applied Botany and Food Quality 80:116–128.
Hawkesford MJ, Kopriva S, De Kok LJ, Eds. 2014. Nutrient Use Efficiency in Plants - Concepts and Approaches. Switzerland: Springer International Publishing.
Haworth M, Moser G, Raschi A, Kammann C, Grünhage L, Müller C. 2016. Long-term (14 years) Free Air Carbone Dioxide Enrichment to 2030 levels does not alter photosynthetic capacity and leaf gas exchange in six temperate grassland species. Functional Plant Biology 43:26–39.
Hinsinger P, Plassard C, Tang C, Jaillard B. 2003. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant and Soil 248:43–59.
Houshmandfar A, Fitzgerald GJ, O´Leary G, Tausz-Posch, S, Fletcher, A, Tausz, M. 2018. The relationship between transpiration and nutrient uptake in wheat changes under elevated atmospheric CO2. Physiologia Plantarum 163:516–529.
Hovenden MJ, Newton PCD, Osanai Y. 2017. Warming has a larger and more persisten effect than elevated CO2 on growing season soil nitrogen availability in a species-rich grassland. Plant Soil 421:417–428.
Jäger H-J, Schmidt SW, Kammann C, Grünhage L, Müller C, Hanewald K. 2003. The University of Giessen Free-Air Carbon Dioxide Enrichment Study: Description of the experimental site and of a new enrichment system. Journal of Applied Botany 77:117–127.
Kammann C, Grünhage L, Grüters U, Janze S, Jäger H-J. 2005. Response of aboveground grassland biomass and soil moisture to moderate long-term CO2 enrichment. Basic and Applied Ecology 6:351–365.
Keidel L, Lenhart K, Moser G, Müller C. 2018. Depth-dependent response of soil aggregates and soil organic carbon content to long-term elevated CO2 in a temperate grassland soil. Soil Biology and Biochemistry 123:145–154.
Leuzinger S, Luo Y, Beier C, Dieleman W, Vicca S, Körner C. 2011. Do global change experiments overestimate impacts on terrestrial ecosystems? Trends in Ecology and Evolution 26:236–241.
Liang J, Qi X, Souza L, Luo Y. 2016. Processes regulating progessive nitrogen limitation under elevated carbon dioxide: a meta-analysis. Biogeosciences 13:2689–2699.
Loladze I. 2002. Rising atmospheric CO2 and human nutrition: toward globally imbalanced plant stoichiometry. Trends in Ecology & Evolution 10:457–461.
Lüscher A, Nösberger J. 1997. Interspecific and intraspecific variability in the response of grasses and legumes to free air CO2 enrichment. Acta Oecologica 18:269–275.
Marschner, H. (2002) Mineral nutrition of higher plants. Academic Press, Elsevier Science Ltd. London.
McCune, B., Grace, J.B. (2002) Analysis of ecological communities. MjM Software Design, Gleneden Beach, Oregon, U.S.A.
McCune, B., Mefford, M.J. (2011) PC-ORD. Multivariate Analysis of Ecological Data. Version 6 MjM Software, Gleneden Beach, Oregon, U.S.A.
Moser G, Gorenflo A, Brenzinger K, Keidel L, Braker G, Marhan S, Clough TJ, Müller C. 2018. Explaining the doubling of N2O emissions under elevated CO2 in the Giessen FACE via in-field 15N tracing. Global Change Biology 24:3897–3910.
Müller C, Rütting T, Abbasi MK, Laughlin RJ, Kammann C, Clough TJ, RR, Sherlock, J, Kattge, HJ, Jäger, CJ, Watson, Stevens RJ. 2009. Effect of elevated CO2 on soil N dynamics in a temperate grassland soil. Soil Biology & Biochemistry 41:1996–2001.
Müller C, Seibert R, Moser G, Zaman M, Schmid T. 2019. Quantifying future climate change impacts on agricultural systems: Results from a long term FACE study on grassland. Soils Newsletter 41(2):6–9.
Myers SS, Zanobetti A, Kloog I, Huybers P, Leaky ADB, Bloom AJ, Carlisle E, Dietterich LH, Fitzgerald G, Hasegawa T, Holbrook NM, Nelson RL, Ottman MJ, Raboy V, Sakai H, Sartor KA, Schwartz J, Seneweera S, Tausz M, Usui Y. 2014. Increasing CO2 threatens human nutrition. Nature 510:139–142.
Natali SM, Sanudo-Wilhelmy SA, Lerdau MT. 2009. Plant and soil mediation of elevated CO2 impacts on trace metals. Ecosystems 12:715–727.
Newton PCD, Lieffering M, Saman WM, Bowatte D, Brock SC, Hunt CL, Theobald PW, Ross DJ. 2010. The rate of progession and stability of progressive nitrogen limitation at elevated atmospheric CO2 in a grazed grassland over 11 years in Free Air CO2 enrichment. Plant Soil 336:433–441.
Newton PCD, Lieffering M, Parsons AJ, Brock SC, Theobald PW, Hunt CL, Luo D, Hovenden MJ. 2014. Selective grazing modifies previously anticipated responses of plant community composition to elevated CO2 in a temperate grassland. Global Change Biology 20:158–169.
Nord EA, Lynch JP. 2009. Plant phenology: a critical controller of soil resource acquisition. Journal of Experimental Botany 7:1927–1937.
Obermeier WA, Lehnert LW, Kammann CI, Müller C, Grünhage L, Luterbacher J, Erbs M, Moser G, Seibert R, Yuan N, Bendix J. 2017. Reduced CO2 fertilization effect in temperate C3 grasslands under more extreme weather conditions. Nature Climate Change 7:137–141.
Reich PB, Hobbie SE, Lee TD, Pastore MA. 2018. Unexpected reversal of C3 and C4 grass response to elevated CO2 during a 20-year field experiment. Science 360(6386):317–320.
Rogers A, Ainsworth EA, Leakey ADB. 2009. Will elevated carbon dioxide concentration amplify the benefits of nitrogen fication in legumes? Plant Physiology 151:1009–1016.
Rütting T. 2017. Nitrogen mineralization, not N2 fixation, alleviates progressive nitrogen limitation – Comment on “Processes regulating progessive nitrogen limitation under elevated carbon dioxide: a meta-analysis” by Liang et al, (2016). Biogeosciences 14:751–754.
Rütting T, Andresen LC. 2015. Nitrogen cycle responses to elevated CO2 depend on ecosystem nutrient status. Nutrient Cyclings in Agro Ecosystems 101:285–294.
Rütting T, Hovenden MJ. 2020. Soil nitrogen cycle unresponsive to decadal long climate change in a Tasmanian grassland. Biogeochemistry 147:99–107.
Safari H, Fricke T, Reddersen B, Möckel T, Wachendorf M. 2016. Comparing mobile and static assessment of biomass in heterogenous grassland with a multi-sensor system. Journal of Sensors and Sensor Systems 5:301–312.
Seibert, R., Donath, T.W., Moser, G., Laser, H., Grünhage, L., Schmid, T., Müller, C. (2021) Effects of long-term CO2 enrichment on forage quality of extensively managed temperate grassland. Agrigulture, Ecosystems and Environment 312, 107347.
Sistla SA, Schimel JP. 2012. Stoichiometric flexibility as a regulator of carbon and nutrient cycling in terrestrial ecosystems under change. New Phytologist 196:68–78.
Smith MR, Myers SS. 2018. Impact of anthropogenic CO2 emissions on global human nutrition. Nature Climate Change 8:834–839.
Tausz-Posch S, Armstrong R, Tausz M. 2014. Nutrient Use and Nutrient Use Efficiency of Crops in a High CO2 Atmosphere. In: Hawkesford MJ, Kopriva S, De Kok LJ, Eds. (2014) Nutrient Use Efficiency in Plants - Concepts and Approaches, . Switzerland: Springer International Publishing. pp 229–252.
van der Maarel E. 1979. Transformation of cover-abundance values in phytosociology and its effects on community similarity. Vegetation 39–2:97–114.
Warton DI, Wright IJ, Falster DS, Westoby M. 2006. Bivariate line-fitting methods for allometry. Biol. Rev. 81:259–291.
Welch RM, Shuman L. 1995. Micronutrient nutrition of plants. Critical Reviews in Plant Science 14:49–82.
White A, Cannell MGR, Friend AD. 2000. CO2 stabilization, climate change and the terrestrial carbon sink. Glob. Chang. Biol. 6:817–833.
WMO (2012) Standardized Precipitation Index User Guide. WMO-No. 1090, ISBN 978–92–63–11091–6.
Yuan ZY, Chen YH. 2015. Decoupling of nitrogen and phosphorus in terrestrial plants associated with global changes. Nature Climate Change 5:465–469.
Yuan N, Moser G, Müller C, Obermeier WA, Bendix J, Lutherbacher J. 2018. Extreme climatic events down-regulate the grassland biomass response to elevated carbon dioxide. Scientific Reports 8:17758.
Acknowledgements
We thank Birte Lenz, JLU-Giessen for her long-term help for plant harvest, sample preparation and C and N analysis, Ralf Lehnart and the Department of Soil Science and Plant Nutrition, Geisenheim University for carrying out the ICP-MS analysis. We thank Ludger Grünhage, Jürgen Franz, Gerlinde Lehr, Gerhard Mayer, Sigfried Schmidt, Jochen Senkbeil, Wolfgang Stein, Nicol Strassila, Till Strohbusch and Andreas Brück at JLU-Giessen for long-term assistance with the management and technical maintenance of the GiFACE site. We are grateful for long-term financial support from the Hessian Agency for Nature Conservation, Environment and Geology (HLNUG), and we acknowledge the funding by the LOEWE excellence cluster FACE2FACE from the Hessian State Ministry of Higher Education, Research and the Arts. We keep a respectful memory of the deceased Professor Dr. Dr. h.c. Hans-Jürgen Jäger, who initiated and sustained the GiFACE experiment.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Ruben Seibert and Louise C. Andresen are joint 1st authorship.
Author contributions: CM, CIK and LCA managed and scheduled the sample analysis. RJL and CJW conducted ICP-MS analysis at AFBI. RS and LCA carried out the statistical analysis of the data and created the graphics; CIK, GM, CM conducted the year-long field work and coordinated the eCO2 experiment. All authors (RS, LCA, KAJ, GM, CIK, NY, JL, RJL, CJW, ME and CM) took part in the planning of this synthesis paper and contributed to the interpretation and discussion of the results and writing of the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
10021_2021_703_MOESM1_ESM.jpg
Figure S1: PCA ordination diagram of the plant nutrient concentrations of N, P, K, Ca, Mg, Zn, and Mn from each year, separated in grasses (circles), forbs (triangles) and legumes (squares), from the two harvests H1 and H2, where H1 eCO2 is red, and aCO2 is blue, and H2 eCO2 is dark red and aCO2 is dark blue. The axes explain 89 % of the total variance
Supplementary file1 (JPG 77 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seibert, R., Andresen, L.C., Jarosch, K.A. et al. Plant Functional Types Differ in Their Long-term Nutrient Response to eCO2 in an Extensive Grassland. Ecosystems 25, 1084–1095 (2022). https://doi.org/10.1007/s10021-021-00703-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-021-00703-y