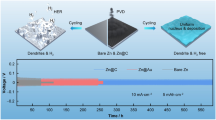
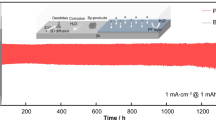
Abstract
Aqueous zinc-ion batteries (AZIBs) are considered promising candidates for scalable and sustainable energy storage devices due to their low cost and high safety advantages. However, the practical application of AZIBs is subject to the limits of the reversibility of the Zn2+ stripping/plating, such as the growth of Zn dendrites, corrosion of Zn anode, and hydrogen evolution reaction (HER). Herein, we propose a solution to the issues of AZIBs by creating a versatile electrolyte via the addition of polyethylene glycol (PEG). PEG additive changes the coordination environment of Zn2+ and disturbs the solvation structure of H2O, which is beneficial to alleviate the side reactions triggered by water molecules in the Zn2+ solvation structure. In addition, the Zn2+ nucleation behavior is optimized by texturing the hexagonal deposition plane and inducing the compact-grained deposition manner to resist corrosion reactions and dendrite aggravation. The solvation structure of Zn2+ is optimized to improve the reversibility of the Zn anode. As a result, improved reversibility of Zn plating/stripping on the Cu-working electrode is achieved by 20% PEG electrolyte with high Coulombic efficiency (CE) of 99.43% at 1 mA cm−2, stably cycled for 750 cycles. Furthermore, the Zn||Zn symmetrical half-cell with 20% PEG electrolyte shows stable plating/stripping overpotentials for more than 1400 h. Finally, the Zn||V2O5 full-cells exhibit excellent long cycling stability for over 180 cycles even at 1000 mA g−1 with a high reversible capacity of 168 mAh g−1. This work provides valuable insights into designing electrolytes for aqueous zinc metal batteries.









Similar content being viewed by others
References
Pan Z, Liu X, Yang J et al (2021) Aqueous rechargeable multivalent metal-ion batteries: advances and challenges. Adv Energy Mater 11:1–24. https://doi.org/10.1002/aenm.202100608
Chen L, An Q, Mai L (2019) Recent advances and prospects of cathode materials for rechargeable aqueous zinc-ion batteries. Adv Mater Interfaces 6:1–24. https://doi.org/10.1002/admi.201900387
Liu H, Wang JG, You Z et al (2021) Rechargeable aqueous zinc-ion batteries: mechanism, design strategies and future perspectives. Mater Today 42:73–98. https://doi.org/10.1016/j.mattod.2020.08.021
Sada K, Darga J, Manthiram A (2023) Challenges and prospects of sodium-ion and potassium-ion batteries for mass production. Adv Energy Mater 13:1–22. https://doi.org/10.1002/aenm.202302321
Li Z, Wei X, Yang Z (2023) Pulsed laser 3D-micro/nanostructuring of materials for electrochemical energy storage and conversion. Prog Mater Sci 133:101052. https://doi.org/10.1016/j.pmatsci.2022.101052
Nimkar A, Bergman G, Ballas E et al (2023) Polyimide compounds for post-lithium energy storage applications. Angew Chem 135(50):e202306904. https://doi.org/10.1002/ange.202306904
Li R, Deng R, Wang Z et al (2023) The challenges and perspectives of developing solid-state electrolytes for rechargeable multivalent battery. J Solid State Electrochem 27:1291–1327. https://doi.org/10.1007/s10008-023-05426-9
Gou L, Zhao W, Li H et al (2024) A facile strategy to unlock the high capacity of vanadium-based cathode for aqueous zinc-ion batteries. J Solid State Electrochem 28:113–123. https://doi.org/10.1007/s10008-023-05673-w
Yin J, Feng X, Gan Z et al (2023) From anode to cell: synergistic protection strategies and perspectives for stabilized Zn metal in mild aqueous electrolytes. Energy storage mater 54:623–640. https://doi.org/10.1016/j.ensm.2022.11.006
Ma L, Zhi C (2021) Zn electrode/electrolyte interfaces of Zn batteries: a mini review. Electrochem commun 122:106898. https://doi.org/10.1016/j.elecom.2020.106898
Zhao T, Wu H, Wen X et al (2022) Recent advances in MOFs/MOF derived nanomaterials toward high-efficiency aqueous zinc ion batteries. Coord Chem Rev 468:214642. https://doi.org/10.1016/j.ccr.2022.214642
Wang F, Zhang J, Lu H et al (2023) Production of gas-releasing electrolyte-replenishing Ah-scale zinc metal pouch cells with aqueous gel electrolyte. Nat Commun 14:1–10. https://doi.org/10.1038/s41467-023-39877-5
Wang J, Qiu H, Zhang Q et al (2023) Eutectic electrolytes with leveling effects achieving high depth-of-discharge of rechargeable zinc batteries. Energy storage mater 58:9–19. https://doi.org/10.1016/j.ensm.2023.03.014
Xu W, Li J, Liao X et al (2023) Fluoride-rich, organic-inorganic gradient interphase enabled by sacrificial solvation shells for reversible zinc metal batteries. J Am Chem Soc 145:22456–22465. https://doi.org/10.1021/jacs.3c06523
Cao Q, Gao Y, Pu J et al (2023) Gradient design of imprinted anode for stable Zn-ion batteries. Nat Commun 14:1–11. https://doi.org/10.1038/s41467-023-36386-3
Lu H, Zhang D, Jin Q et al (2023) Gradient electrolyte strategy achieving long-life zinc anodes. Adv Mater 35:1–37. https://doi.org/10.1002/adma.202300620
Zheng J, Cao Z, Ming F et al (2022) Preferred orientation of TiN coatings enables stable zinc anodes. ACS Energy Lett 7:197–203. https://doi.org/10.1021/acsenergylett.1c02299
Javed MS, Najam T, Hussain I et al (2023) Fundamentals and scientific challenges in structural design of cathode materials for zinc-ion hybrid supercapacitors. Adv Energy Mater 13:1–49. https://doi.org/10.1002/aenm.202202303
Deng W, Xu Z, Wang X (2022) High-donor electrolyte additive enabling stable aqueous zinc-ion batteries. Energy storage mater 52:52–60. https://doi.org/10.1016/j.ensm.2022.07.032
Ma L, Vatamanu J, Hahn NT et al (2022) Highly reversible Zn metal anode enabled by sustainable hydroxyl chemistry. Proc Natl Acad Sci U S A 119:1–9. https://doi.org/10.1073/pnas.2121138119
Han M, Li TC, Chen X, Yang HY (2024) Electrolyte modulation strategies for low-temperature Zn batteries. Small 20:2304901. https://doi.org/10.1002/smll.202304901
Li D, Cao L, Deng T et al (2021) Design of a solid electrolyte interphase for aqueous Zn batteries. Angewandte Chemie - International Edition 60:13035–13041. https://doi.org/10.1002/anie.202103390
Hao J, Li X, Zhang S et al (2020) Designing dendrite-free zinc anodes for advanced aqueous zinc batteries. Adv Funct Mater 30:1–10. https://doi.org/10.1002/adfm.202001263
Xie W, Zhu K, Yang H, Yang W (2023) Advancements in achieving high reversibility of zinc anode for alkaline zinc-based batteries. Adv Mater 36:202306154. https://doi.org/10.1002/adma.202306154
Cao Z, Zhang H, Song B et al (2023) Angstrom-level ionic sieve 2D-MOF membrane for high power aqueous zinc anode. Adv Funct Mater 33:1–12. https://doi.org/10.1002/adfm.202300339
Zeng Y, Pei Z, Luan D, Lou XWD (2023) Atomically dispersed zincophilic sites in N, P-codoped carbon macroporous fibers enable efficient Zn metal anodes. J Am Chem Soc 145:12333–12341. https://doi.org/10.1021/jacs.3c03030
Guo X, Zhang Z, Li J et al (2021) Alleviation of dendrite formation on zinc anodes via electrolyte additives. ACS Energy Lett 6:395–403. https://doi.org/10.1021/acsenergylett.0c02371
Chen S, Ying Y, Ma L et al (2023) An asymmetric electrolyte to simultaneously meet contradictory requirements of anode and cathode. Nat Commun 14:1–11. https://doi.org/10.1038/s41467-023-38492-8
Li Z, Liao Y, Wang Y et al (2023) A co-solvent in aqueous electrolyte towards ultralong-life rechargeable zinc-ion batteries. Energy storage mater 56:174–182. https://doi.org/10.1016/j.ensm.2023.01.020
Wan J, Wang R, Liu Z et al (2023) A double-functional additive containing nucleophilic groups for high-performance Zn-ion batteries. ACS Nano 17(2):1610–1621. https://doi.org/10.1021/acsnano.2c11357
Zhong Y, Xie X, Zeng Z et al (2023) Triple-function hydrated eutectic electrolyte for enhanced aqueous zinc batteries. Angewandte Chemie - International Edition 62(40):e202310577. https://doi.org/10.1002/anie.202310577
Wang Y, Wang T, Bu S et al (2023) Sulfolane-containing aqueous electrolyte solutions for producing efficient ampere-hour-level zinc metal battery pouch cells. Nat Commun 14:1–13. https://doi.org/10.1038/s41467-023-37524-7
Zhu J, Yang M, Hu Y et al (2023) The construction of binary phase electrolyte interface for highly stable zinc anodes. Adv Mater 36(3):2304426. https://doi.org/10.1002/adma.202304426
You C, Wu R, Yuan X et al (2023) Inexpensive electrolyte with double-site hydrogen bonding and regulated Zn2+ solvation structure for aqueous Zn-ion batteries capable of high-rate and ultra-long low-temperature operation. Energy Environ Sci 16:5096–5107. https://doi.org/10.1039/d3ee01741a
Han D, Sun T, Zhang RC et al (2022) Eutectic electrolytes with doubly-bound water for high-stability zinc anodes. Adv Funct Mater 32(52):2209065. https://doi.org/10.1002/adfm.202209065
Cai Z, Wang J, Lu Z et al (2022) Ultrafast metal electrodeposition revealed by in situ optical imaging and theoretical modeling towards fast-charging Zn battery chemistry. Angewandte Chemie - International Edition 61:1–8. https://doi.org/10.1002/anie.202116560
Wang F, Borodin O, Gao T et al (2018) Highly reversible zinc metal anode for aqueous batteries. Nat Mater 17:543–549. https://doi.org/10.1038/s41563-018-0063-z
Zhang C, Shin W, Zhu L et al (2021) The electrolyte comprising more robust water and superhalides transforms Zn-metal anode reversibly and dendrite-free. Carbon energy 3:339–348. https://doi.org/10.1002/cey2.70
Feng X, Li P, Yin J et al (2023) Enabling highly reversible Zn anode by multifunctional synergistic effects of hybrid solute additives. ACS Energy Lett 8:1192–1200. https://doi.org/10.1021/acsenergylett.2c02455
Hao J, Yuan L, Ye C et al (2021) Boosting zinc electrode reversibility in aqueous electrolytes by using low-cost antisolvents. Angewandte Chemie - International Edition 60:7366–7375. https://doi.org/10.1002/anie.202016531
Gomez Vazquez D, Pollard TP, Mars J et al (2023) Creating water-in-salt-like environment using coordinating anions in non-concentrated aqueous electrolytes for efficient Zn batteries. Energy Environ Sci 16:1982–1991. https://doi.org/10.1039/d3ee00205e
Chen Y, Guo S, Qin L et al (2022) Low current-density stable zinc-metal batteries via aqueous/organic hybrid electrolyte. Batter Supercaps 5(5):e202200001. https://doi.org/10.1002/batt.202200001
Ming F, Liang H, Lei Y et al (2018) Layered MgxV2O5·nH2O as cathode material for high performance aqueous zinc ion batteries. ACS Energy Lett 3:2602–2609. https://doi.org/10.1021/acsenergylett.8b01423
Chen X, Wang L, Li H et al (2019) Porous V2O5 nanofibers as cathode materials for rechargeable aqueous zinc-ion batteries. J Energy Chem 38:20–25. https://doi.org/10.1016/j.jechem.2018.12.023
Xu D, Wang H, Li F et al (2019) Conformal conducting polymer shells on V2O5 nanosheet arrays as a high-rate and stable zinc-ion battery cathode. Adv Mater Interfaces 6(2):1801506. https://doi.org/10.1002/admi.201801506
Li R, Xing F, Li T et al (2021) Intercalated polyaniline in V2O5 as a unique vanadium oxide bronze cathode for highly stable aqueous zinc ion battery. Energy Storage Mater 38:590–598. https://doi.org/10.1016/j.ensm.2021.04.004
Luo X, Zhou M, Luo Z et al (2023) Regulation of desolvation process and dense electrocrystalization behavior for stable Zn metal anode. Energy Storage Mater 57:628–638. https://doi.org/10.1016/j.ensm.2023.03.002
Chen W, Guo S, Qin L et al (2022) Hydrogen bond-functionalized massive solvation modules stabilizing bilateral interfaces. Adv Funct Mater 32:1–12. https://doi.org/10.1002/adfm.202112609
Wang F, Lu H, Li H et al (2022) Demonstrating U-shaped zinc deposition with 2D metal-organic framework nanoarrays for dendrite-free zinc batteries. Energy Storage Mater 50:641–647. https://doi.org/10.1016/j.ensm.2022.06.005
Li TC, Lin C, Luo M et al (2023) Interfacial molecule engineering for reversible Zn electrochemistry. ACS Energy Lett 8:3258–3268. https://doi.org/10.1021/acsenergylett.3c00859
Liu Z, Guo Z, Fan L et al (2024) Construct robust epitaxial growth of (101) textured zinc metal anode for long life and high capacity in mild aqueous zinc-ion batteries. Adv Mater 36(5):2305988. https://doi.org/10.1002/adma.202305988
Dou X, Xie X, Liang S, Fang G (2024) Low-current-density stability of vanadium-based cathodes for aqueous zinc-ion batteries. Sci Bull. https://doi.org/10.1016/j.scib.2024.01.029
Wang Z, Zhou M, Qin L et al (2022) Simultaneous regulation of cations and anions in an electrolyte for high-capacity, high-stability aqueous zinc-vanadium batteries. eScience 2:209–218. https://doi.org/10.1016/j.esci.2022.03.002
Acknowledgements
This work was financially supported by the Natural Science Foundation of Jiangsu Province (BK20220651), the Natural Science Foundation of the Jiangsu Higher Education Institution of China (21KJB480010), and the National Natural Science Foundation of China (51672114). This work was also supported by the Scientific Research Foundation of Jiangsu University of Science and Technology (1112932004).
Funding
Natural Science Foundation of Jiangsu Province,BK20220651,Xiaogang Li,Natural Science Research of Jiangsu Higher Education Institutions of China,21KJB480010,Xiaogang Li,National Natural Science Foundation of China,51672114,Aihua Yuan
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Zhou, Y., Tu, H. et al. Improving Zn anode electrochemical reversibility via crystallographic plane regulation by polyethylene glycol electrolyte additive. J Solid State Electrochem (2024). https://doi.org/10.1007/s10008-024-05901-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10008-024-05901-x