Abstract
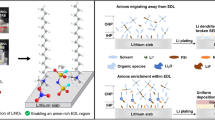
Aqueous zinc-ion batteries are considered promising energy storage devices due to their low cost, high capacity, and high safety. However, the application of aqueous zinc-ion batteries is subject to the limits of Zn anode reversibility, such as the formation of Zn dendrites, Zn anode corrosion, and hydrogen evolution reaction. In this study, we reported an electrolyte additive ethylene glycol monomethyl ether (MOE) with a modulated electrolyte solvation structure, which can suppress the growth of Zn dendrites and enhance the reversibility of Zn chemistry. The MOE molecular shows a higher electron cloud density of oxygen atoms, which enhances the strength of H–O covalent bonds. Therefore, MOE can break the interaction between H2O and H2O and the interaction between H2O and Zn2+. The solvation structure of Zn2+ is optimized to improve the reversibility of the Zn anode. As a consequence, the reversibility of Zn plating/stripping is promoted by 20% MOE-modified electrolyte with high CE of 99.4% at the current density of 1 mA cm−2, stably cycled for 2100 cycles. The Zn||Zn symmetrical cells can keep dendrite-free cycling for more than 4000 h in the same target electrolyte. In addition, the Zn||V2O5 cell can exhibit excellent long cycling stability for over 1000 cycles even at 5 A g−1 with the reversible capacity of 100 mAh g−1. This work provides a universal additive strategy to design versatile electrolytes for aqueous zinc-ion batteries.







Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Dai Y, Zhang C, Li J et al (2024) Inhibition of vanadium cathodes dissolution in aqueous Zn-ion batteries. Adv Mater 2310645:1–9. https://doi.org/10.1002/adma.202310645
Xie W, Zhu K, Yang H, Yang W (2023) Advancements in achieving high reversibility of zinc anode for alkaline zinc-based batteries. Adv Mater 36:2306154. https://doi.org/10.1002/adma.202306154
Chen H, Li X, Fang K et al (2023) Aqueous zinc-iodine batteries: from electrochemistry to energy storage mechanism. Adv Energy Mater 13:2302187. https://doi.org/10.1002/aenm.202302187
Wei T, Ren Y, Wang Y et al (2023) Addition of dioxane in electrolyte promotes (002)-textured zinc growth and suppressed side reactions in zinc-ion batteries. ACS Nano 17:3765–3775. https://doi.org/10.1021/acsnano.2c11516
Cao Q, Gao Y, Pu J et al (2023) Gradient design of imprinted anode for stable Zn-ion batteries. Nat Commun 14:1–11. https://doi.org/10.1038/s41467-023-36386-3
Yang Q, Li Q, Liu Z et al (2020) Dendrites in Zn-based batteries. Adv Mater 32:1–32. https://doi.org/10.1002/adma.202001854
Bai S, Huang Z, Liang G et al (2023) Electrolyte additives for stable Zn anodes. Advanced Science 2304549:1–20. https://doi.org/10.1002/advs.202304549
Xu W, Li J, Liao X et al (2023) Fluoride-rich, organic-inorganic gradient interphase enabled by sacrificial solvation shells for reversible zinc metal batteries. J Am Chem Soc 145:22456–22465. https://doi.org/10.1021/jacs.3c06523
Lu H, Zhang D, Jin Q et al (2023) Gradient electrolyte strategy achieving long-life zinc anodes. Adv Mater 35:1–37. https://doi.org/10.1002/adma.202300620
Ma L, Vatamanu J, Hahn NT et al (2022) Highly reversible Zn metal anode enabled by sustainable hydroxyl chemistry. Proc Natl Acad Sci U S A 119:1–9. https://doi.org/10.1073/pnas.2121138119
Han M, Li TC, Chen X, Yang HY (2023) Electrolyte modulation strategies for low-temperature Zn batteries. Small 2304901:1–16. https://doi.org/10.1002/smll.202304901
Meng C, He WD, Tan H et al (2023) A eutectic electrolyte for an ultralong-lived Zn//V2O5 cell: an in situ generated gradient solid-electrolyte interphase. Energy Environ Sci 16:3587–3599. https://doi.org/10.1039/d3ee01447a
Li D, Cao L, Deng T et al (2021) Design of a solid electrolyte interphase for aqueous Zn batteries. Angew Chem Int Ed 60:13035–13041. https://doi.org/10.1002/anie.202103390
Zeng X, Xie K, Liu S et al (2021) Bio-inspired design of an in situ multifunctional polymeric solid-electrolyte interphase for Zn metal anode cycling at 30 mA cm-2 and 30 mA h cm-2. Energy Environ Sci 14:5947–5957. https://doi.org/10.1039/d1ee01851e
Di S, Nie X, Ma G et al (2021) Zinc anode stabilized by an organic-inorganic hybrid solid electrolyte interphase. Energy Storage Mater 43:375–382. https://doi.org/10.1016/j.ensm.2021.09.021
Liu C, Luo Z, Deng W et al (2021) Liquid alloy interlayer for aqueous zinc-ion battery. ACS Energy Lett 6:675–683. https://doi.org/10.1021/acsenergylett.0c02569
Wang SB, Ran Q, Yao RQ et al (2020) Lamella-nanostructured eutectic zinc–aluminum alloys as reversible and dendrite-free anodes for aqueous rechargeable batteries. Nat Commun 11:1–9. https://doi.org/10.1038/s41467-020-15478-4
Wang F, Zhang J, Lu H et al (2023) Production of gas-releasing electrolyte-replenishing Ah-scale zinc metal pouch cells with aqueous gel electrolyte. Nat Commun 14:1–10. https://doi.org/10.1038/s41467-023-39877-5
Wang F, Sun W, Shadike Z et al (2018) How water accelerates bivalent ion diffusion at the electrolyte/electrode interface. Angew Chem 130:12154–12157. https://doi.org/10.1002/ange.201806748
Chen H, Dai C, Xiao F et al (2022) Reunderstanding the reaction mechanism of aqueous Zn-Mn battery in sulfate electrolytes: role of the zinc sulfate hydroxide. Adv Mater 34:2109092. https://doi.org/10.1002/adma.202109092
Xie J, Liang Z, Lu YC (2020) Molecular crowding electrolytes for high-voltage aqueous batteries. Nat Mater 19:1006–1011. https://doi.org/10.1038/s41563-020-0667-y
Qin Y, Liu P, Zhang Q et al (2020) Advanced filter membrane separator for aqueous zinc-ion batteries. Small 16:1–10. https://doi.org/10.1002/smll.202003106
Fang Y, Xie X, Zhang B et al (2021) Regulating zinc deposition behaviors by the conditioner of PAN separator for zinc-ion batteries. Adv Funct Mater 32:2109671. https://doi.org/10.1002/adfm.202109671
Cao P, Zhou H, Zhou X et al (2022) Stabilizing zinc anodes by a cotton towel separator for aqueous zinc-ion batteries. ACS Sustain Chem Eng 10:8350–8359. https://doi.org/10.1021/acssuschemeng.2c01133
Wu B, Wu Y, Lu Z et al (2021) A cation selective separator induced cathode protective layer and regulated zinc deposition for zinc ion batteries. J Mater Chem A 9:4734–4743. https://doi.org/10.1039/d0ta11841a
Zhang F, Huang F, Huang R et al (2023) Hierarchical porous separator with excellent isotropic modulus enabling homogeneous Zn2+ flux for stable aqueous Zn battery. Sci China Mater 66:982–991. https://doi.org/10.1007/s40843-022-2239-8
Wang Y, Wang T, Bu S et al (2023) Sulfolane-containing aqueous electrolyte solutions for producing efficient ampere-hour-level zinc metal battery pouch cells. Nat Commun 14:1–13. https://doi.org/10.1038/s41467-023-37524-7
Zhu J, Yang M, Hu Y et al (2023) The construction of binary phase electrolyte interface for highly stable zinc anodes. Adv Mater 36:2304426. https://doi.org/10.1002/adma.202304426
You C, Wu R, Yuan X et al (2023) An inexpensive electrolyte with double-site hydrogen bonding and regulated Zn 2+ Solvation structure for aqueous Zn-ion batteries capable of high-rate and ultra-long low-temperature operation. Energy Environ Sci 16:5096–5107. https://doi.org/10.1039/d3ee01741a
Han D, Sun T, Zhang RC et al (2022) Eutectic electrolytes with doubly-bound water for high-stability zinc anodes. Adv Funct Mater 32:2209065. https://doi.org/10.1002/adfm.202209065
Cai Z, Wang J, Lu Z et al (2022) Ultrafast metal electrodeposition revealed by in situ optical imaging and theoretical modeling towards fast-charging Zn battery chemistry. Angew Chem Int Ed 61:1–8. https://doi.org/10.1002/anie.202116560
Wang F, Borodin O, Gao T et al (2018) Highly reversible zinc metal anode for aqueous batteries. Nat Mater 17:543–549. https://doi.org/10.1038/s41563-018-0063-z
Zhang C, Shin W, Zhu L et al (2021) The electrolyte comprising more robust water and superhalides transforms Zn-metal anode reversibly and dendrite-free. Carbon Energy 3:339–348. https://doi.org/10.1002/cey2.70
Deng W, Xu Z, Wang X (2022) High-donor electrolyte additive enabling stable aqueous zinc-ion batteries. Energy Storage Mater 52:52–60. https://doi.org/10.1016/j.ensm.2022.07.032
Feng X, Li P, Yin J et al (2023) Enabling highly reversible Zn anode by multifunctional synergistic effects of hybrid solute additives. ACS Energy Lett 8:1192–1200. https://doi.org/10.1021/acsenergylett.2c02455
Hao J, Yuan L, Ye C et al (2021) Boosting zinc electrode reversibility in aqueous electrolytes by using low-cost antisolvents. Angew Chem Int Ed 60:7366–7375. https://doi.org/10.1002/anie.202016531
Gomez Vazquez D, Pollard TP, Mars J et al (2023) Creating water-in-salt-like environment using coordinating anions in non-concentrated aqueous electrolytes for efficient Zn batteries. Energy Environ Sci 16:1982–1991. https://doi.org/10.1039/d3ee00205e
Tang X, Wang P, Bai M et al (2021) Unveiling the reversibility and stability origin of the aqueous V2O5–Zn batteries with a ZnCl2 “water-in-salt” electrolyte. Adv Sci 8:2–11. https://doi.org/10.1002/advs.202102053
Wang X, Li Y, Das P et al (2020) Layer-by-layer stacked amorphous V2O5/Graphene 2D heterostructures with strong-coupling effect for high-capacity aqueous zinc-ion batteries with ultra-long cycle life. Energy Storage Mater 31:156–163. https://doi.org/10.1016/j.ensm.2020.06.010
Li Z, Ganapathy S, Xu Y et al (2019) Mechanistic insight into the electrochemical performance of Zn/VO2 batteries with an aqueous ZnSO4 electrolyte. Adv Energy Mater 9:1–10. https://doi.org/10.1002/aenm.201900237
Wu M, Wang X, Zhang F et al (2024) Highly reversible and stable Zn metal anodes realized using a trifluoroacetamide electrolyte additive. Energy Environ Sci 17:619–629. https://doi.org/10.1039/d3ee03040g
Hao J, Yuan L, Johannessen B et al (2021) Studying conversion mechanism to broaden cathode options in aqueous Zn-ion batteries. Angew Chem Int Ed 60:25114–25121. https://doi.org/10.1002/anie.202111398
Miao Z, Liu Q, Wei W et al (2022) Unveiling unique steric effect of threonine additive for highly reversible Zn anode. Nano Energy 97:107145. https://doi.org/10.1016/j.nanoen.2022.107145
Wu F, Chen Y, Chen Y et al (2022) Achieving highly reversible zinc anodes via N, N-dimethylacetamide enabled Zn-ion solvation regulation. Small 18:1–9. https://doi.org/10.1002/smll.202202363
Wei T, Peng Y, Mo L et al (2022) Modulated bonding interaction in propanediol electrolytes toward stable aqueous zinc-ion batteries. Sci China Mater 65:1156–1164. https://doi.org/10.1007/s40843-021-1841-5
Qin R, Wang Y, Zhang M et al (2021) Tuning Zn2+ coordination environment to suppress dendrite formation for high-performance Zn-ion batteries. Nano Energy 80:105478. https://doi.org/10.1016/j.nanoen.2020.105478
Yang H, Qiao Y, Chang Z et al (2021) Reducing water activity by zeolite molecular sieve membrane for long-life rechargeable zinc battery. Adv Mater 33:2102415. https://doi.org/10.1002/adma.202102415
Wang X, Tian Y, Yang K et al (2024) Sandwich-structured anode enables high stability and enhanced zinc utilization for aqueous Zn-ion batteries. Energy Storage Mater 64:103078. https://doi.org/10.1016/j.ensm.2023.103078
Ciurduc DE, de la Cruz C, Patil N et al (2023) An improved PEG-based molecular crowding electrolyte using Zn(TFSI)2 vs. Zn(OTf)2 for aqueous Zn//V2O5 battery. Mater Today Energy 36:101339. https://doi.org/10.1016/j.mtener.2023.101339
Hu P, Zhu T, Ma J et al (2019) Porous V2O5 microspheres: a high-capacity cathode material for aqueous zinc-ion batteries. Chem Commun 55:8486–8489. https://doi.org/10.1039/c9cc04053f
Funding
This work was financially supported by the Natural Science Foundation of Jiangsu Province (BK20220651), Natural Science Foundation of the Jiangsu Higher Education Institution of China (21KJB480010), and National Natural Science Foundation of China (51672114). The work was also supported by the Scientific Research Foundation of Jiangsu University of Science and Technology (1112932004).
Author information
Authors and Affiliations
Contributions
XG Li conceived and wrote the main manuscript text; H Tu completed the experiment, physical characterization, and electrochemical tests; R Wu, ZN Wang, and YH Zhou assisted the experiment and tests; YJ Zong, YK Lu, L Qian, YX Zhang, and SY Song provided suggestions for the manuscript; CF Meng provided suggestions for the FTIR and SEM characterization of samples; AH Yuan provided funding and suggestions for the revisions. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Tu, H., Wu, R. et al. Boosting Zn anode reversibility via electrolyte solvation structure mediation by ethylene glycol monomethyl ether additive. Ionics 30, 2665–2676 (2024). https://doi.org/10.1007/s11581-024-05486-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-024-05486-5