Abstract
The electro-Fenton process combined with a boron-doped diamond-positive electrode in a one-compartment cell has shown efficient degradation of Cartap (95% in Padan 95SP) by hydroxyl radicals (•OH) generated in the electro-Fenton and the electrochemical oxidation processes. The influence of added NaOCl in a pretreatment step, effects of H2O2 concentration, Fe2+-ion addition, presence of further metals acting as co-catalysts, and solution pH on the efficiency of Cartap degradation were studied. The concentration of Cartap was determined by UV-vis spectroscopy according to the 5,5-dithiobis-(2-nitrobenzoic acid) procedure. The efficiency reaches approximately 80% when measured as total carbon concentration decrease, even with increased concentrations of H2O2, Fe2+, or metal ions added as co-catalyst. This limitation is presumably due to recalcitrant intermediates, which cannot be destroyed by •OH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Padan 95SP containing 95%wt. Cartap hydrochlorideFootnote 1 (CH) is used in agriculture in Vietnam as a popular pesticide generally assumed to be of low toxicity [1], for further human-health-related information see [2]. Cartap in its hydrochloride form is highly water-soluble causing toxicity in agricultural waste and runoff water. Due to its high insecticidal activity, Cartap is widely used all over the world [3]. In the Mekong Delta (Vietnam), Cartap constitutes about 19% of the insecticide usage in rice and rice-fish farms [4]. In China, Japan, and Korea, it is also one of the most frequently used pesticides for pest control [5, 6]. Excessive use of Cartap and its commercial preparations as well as of related compounds in tea production has been noted [7]. Somewhat misleadingly, nereistoxin—the molecule actually active as pesticide but hardly soluble in water and thus difficult to apply directly—has been called an analogue of Cartap. As shown below, it is a hydrolysis product of Cartap. Details of the hydrolysis as a function of pH and of the toxicity and biological activity of observed intermediates have been reported elsewhere [8]. The extensive application of Cartap as the main compound in pesticides may easily cause environmental pollution and hazards during usage. To avoid further accumulation of Cartap and its hydrolysis and decomposition compounds in aqueous environments and to destroy these contaminants in water, it is necessary to study options to remove them from water.
In advanced oxidation processes (AOPs)Footnote 2, •OH radicals or other highly reactive (i.e., oxidative) species are generated by various means (e.g., electrochemically, by decomposition of H2O2, etc.) to react with organic species in wastewater [9,10,11,12,13]. As a result, the organic species are decomposed into smaller molecular products or finally mineralized into CO2, H2O, and inorganic ions. The recent use of boron-doped diamond (BDD) thin-film electrodes in anodic oxidation reactions has strongly increased interest in their application in water remediation by AOPs. This electrochemical technique is based on the oxidation of organic pollutants by •OH radicals generated by electrooxidation of water according to Eq. 1:
As another option, degradation of Cartap and other pollutants by a simple Fenton process has been examined elsewhere [5]. At optimized reaction conditions, 80% of the initial Cartap could be destroyed, but significant and stable concentrations of degradation products were noticed. Various organic (formic, acetic, and propionic acid) and inorganic (sulfuric and nitric acid) acids were identified as intermediates and/or products. A possible reaction scheme with various pathways was suggested (see also Fig. 12).
In the Fenton process, the •OH radicals are produced by electron transfer between hydrogen peroxide (H2O2) and ferrous ions (Fe2+) in acidic solution (Eq. 2) [14].
Further transition metal ions to be used instead of ferrous ions have been examined previously [15]. A relative rating has been concluded for pH = 7 with Cu(II) causing the highest and Ni(II) the lowest rate of formation of •OH radicals. Fe(III) showed an intermediate activity.
Electrochemical regeneration of Fe2+ ions by electroreduction of Fe3+ proceeds in the so-called electro-Fenton process developed in order to get higher efficiency than with the plain Fenton process [11, 16,17,18,19,20,21,22,32]. Instead of Fe2+ ions, further transition metal ions have been examined [33] Cr(III) was found to be most effective and La(II) least effective. The sequence of activities was established as Cr(III) > Ce(III) > Cu(II) > Co(II) > Fe(II) Ni(II) > La(II). In addition, hydrogen peroxide can be generated by electroreduction of dioxygen at a gas-fed oxygen-consuming electrode; sometimes this feature of in situ generation of hydrogen peroxide is considered most typical of the electro-Fenton process. In the present study, we have omitted this part for simplicity and because of lacking access to dioxygen-consuming gas diffusion electrodes. Instead, we have added hydrogen peroxide to the reactant solution. Accordingly, this approach might be called a modified electro-Fenton process. The electro-Fenton process is enhanced in our setup by using a one-compartment cell where pollutants can be simultaneously oxidized by •OH produced at the anode (Eq. (1)) and where said regeneration proceeds at the cathode [16, 26, 27, 30, 34].
Photocatalytic oxidative degradation of Cartap hydrochloride with TiO2 coated on a ceramic support has been reported [6]. At low Cartap concentrations (50 to 100 mg/L), degradation proceeded to nitrate as one end product (see below).
Degradation of Cartap with a simple but highly effective oxidant like NaOCl has not been tested before; we have examined a pretreatment of a Cartap-containing test solution with NaOCl as a conceivable option to improve overall efficiency of the treatment proposed here.
Here we report a detailed study of the degradation of Cartap in an aqueous solution of 700 mg L−1 Padan 95SP (using the molar mass of Cartap of 273.8 g mol−1 and neglecting the unknown added ingredients in Padan 95SP, this is equivalent to a concentration of 2.6 mM) with 0.05 M Na2SO4 as supporting electrolyte by such combined process at the constant current density of j = 20 mA cm−2 (this value has been established elsewhere as being optimal [35]) using a one-compartment cell. The effects of NaOCl, H2O2 concentration, Fe2+, and co-catalyst metal ion addition, and the pH value on the degradation rate in this combined process were investigated. An attempt was made to identify by-products by high performance liquid chromatography (HPLC); a general reaction pathway for Cartap mineralization is proposed.
Materials and methods
Chemicals
The commercial pesticide Padan 95SP (95% Cartap) was purchased from the Japanese Sumitomo Chemical company branch in Vietnam. Ellman’s reagent (5,5′-dithiobis(2-nitrobenzoic acid, DTNB) purchased from Sigma-Aldrich was used to determine the content of Cartap in Padan 95SP after 5-, 15-, 30-, 60-, 90-, and 120-min electrochemical treatment. Chemicals (AgCl, HCl, H2SO4, BaCl2·2H2O, NaNO3, Na2CO3, Na2SO4, CH3OH, K2Cr2O7, H3PO4, K2CrO4, CH3COOH, C2H5OH, glycerol, boric acid) were obtained from Sigma-Aldrich and Merck. Solutions were prepared using deionized ultrapure water (Seralpur Pro 90 C).
Electrolysis system
Bulk electrolysis was carried out at room temperature (22 °C) in a 400-mL one-compartment cell. BDD electrode was used as the working electrode with 3.8-cm2 circular exposed surface area; the thickness of the diamond coating was 2.5–3 μm. A platinum foil counter electrode and a Ag/AgCl (sat. KCl) reference electrode were used. The electrolyte solution (250 mL) was continuously stirred by a magnetic bar throughout the process. Before the experiments were started, the BDD electrode was subjected to ultrasound for 5 min to remove contaminants and washed with ultrapure water (Seralpur Pro 90 C). The platinum counter electrode was washed with ultrapure water, too. A Padan 95SP concentration of 700 mg L−1 was used. The pH value of the solution was adjusted by H2SO4 and NaOH and measured with a pH meter. The total time of the electrolysis was 120 min.
Determination of Cartap content according to the 5,5-dithiobis-(2-nitrobenzoic acid) procedure [8, 36, 37]
Cartap hydrolyzes in water at a rate strongly dependent on pH (for details see [8]) yielding the active pesticide agent nereistoxin (Fig. 1). The −SCO(NH2) group(s) of Cartap react(s) with DTNB generating the yellow 3-carboxy-4-nitrophenylthiolate anion [38] (Fig. 2), which is detected at 412-nm wavelength using UV-vis spectroscopy. Nereistoxin (NTX) (Fig. 1), which lacks a free thiol group, does not react with DTNB.
According to [8], 0.2 mL test solution and 0.8 mL DTNB solution (1 g L−1) were added to 4 mL of buffer solution (pH = 9). After 1-h reaction time, a UV-vis spectrum was recorded. The calibration curve for Cartap was built up according to the procedure as described above [36].
Analytical methods
Before analysis, all samples taken from electrolysis solutions were filtered using filter paper with a pore size of 2.5 μm. Just two drops of 2 M NaOH were added to the samples to stop the Fenton reaction (The Fenton reaction slows down with increasing pH value, it is practically inhibited when pH > 10. The precipitation of Fe(II) and Fe(III) hydroxide slows down the reaction to a standstill. The particularly low solubility of the latter hydroxide inhibits the electrochemical regeneration of Fe(II) ions.). Solution samples were withdrawn, and the content of Cartap was immediately determined (according to the procedure in section 2.2) by UV-vis after filtration. Sample vessels were kept closed at all times to avoid absorption of carbon dioxide from ambient air possibly resulting in artificially increased carbon content values. The content of Cartap was determined from the maximum absorption at the peak in the UV-vis spectrum of the 3-carboxy-4-nitrophenylthiolate anion at λ = 412 nm. Total carbon content of solutions was determined by standard NPOC (nonpurgeable organic carbon) method using a multi N/C 3100 (Analytik Jena) TOC analyzer. Because necessary precautions were taken to exclude carbon from other sources (e.g., air), the determined carbon content may be called following total organic carbon (TOC) for simplicity.
The products formed during electrochemical oxidation (bulk electrolysis) were analyzed using HPLC (model KNAUER Smartline). A chromatographic column Eurospher 100 5 C8 column (250 × 4.6 mm) and a detector DAD 200–800 nm were used. The mobile phase consisted of 85 vol% water and 15 vol% acetonitrile with the flow rate of 1 mL min−1 at 25 °C. The injection volume was 20 μL, and the working wavelength for quantitative analysis was 210 nm.
The hydrogen peroxide concentration was analyzed using the titanium sulfate spectrophotometric method [39]. The concentration of ClO− (“active chlorine”)/HOCl in the samples was measured by the iodometric method [40].
The mineralization of the organic species in the examined solutions was followed by measuring the decrease of TOC again with the instrument mentioned above.
The following reaction for complete mineralization assuming electrooxidation of Cartap is proposed:
With NaOCl as oxidant (with HClO + H+ + 2 e− → Cl− + H2O), the following equation can be assumed:
With H2O2 as oxidant, the following equation can be assumed:
Results and discussion
Padan 95SP degradation with NaOCl
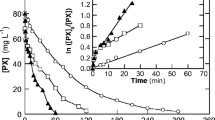
The removal capability of sodium hypochlorite was tested with a solution containing 700 mg L−1 Padan 95SP (equivalent to TOC = 215 mg L−1). Based on Eq. 3b, a concentration of NaOCl c = 0.054 M would be needed at least for the described complete mineralization reaction. Mineralization, possibly incomplete, expressed both in terms of remaining Cartap as well as of TOC (see Fig. 4 and its inset) may proceed. Even at the highest concentration of NaOCl, complete mineralization cannot be expected. Measured concentrations of NaOCl in solutions with different starting concentrations are shown in Fig. 3; progress of Cartap degradation is displayed in Fig. 4. A fast decrease of the concentration of NaOCl immediately after addition before the first determination 2 min after mixing is followed by a more slowly decrease during the following time of reaction (see Fig. 3). The solutions were not stirred; thus, at low concentrations of NaOCl, diffusion control may become dominant with growing reaction time resulting in a slower decrease.
Figure 4 shows that Cartap decomposition starts immediately after adding NaOCl but there is no more decomposition after 2 min (in case of low concentrations 8 and 12 mM NaOCl) although some NaOCl still remains in solution (as can be seen in Fig. 3). Cartap remains in treated solutions at 20 and 45% of its initial value with concentrations of NaOCl 12 mM and 8 mM; meanwhile, it is totally decomposed after 2 min with 15 mM and 22 mM NaOCl.
Thus, 15 mM NaOCl is sufficient to remove Cartap completely in a test solution containing 700 mg L−1 Padan 95SP. In this case, TOC remained at 78.33% (see insert of Fig. 4). It can be concluded that NaOCl is a very efficient oxidizing agent which needs only a short time to decompose Cartap by releasing free chlorine (HOCl and ClO− “active chlorine”). The reactions are shown in Eqs. (4) and (5), see also Eq. 3b.
Effect of Electro-Fenton treatment
Electro-Fenton oxidation treatment was tested by electrolyzing 700 mg L−1 Padan 95SP solutions (equivalent to 215 mg L−1 of TOC) of pH = 3 at j = 20 mA cm−2 at room temperature for 120 min with the addition of H2O2 (instead of feeding the cathode with O2 for H2O2 generation). Cartap is decomposed almost quantitatively after 5 min at various H2O2 concentrations; 0.2 M H2O2 can be chosen as a sufficient value for Cartap degradation (see Fig. 5). Based on Eq. 3c, a concentration of H2O2 c = 0.054 M would be needed at least for the described mineralization reaction. The very rapid, almost complete consumption of H2O2 at its initial concentration c = 0.05 M supports this estimate.
Figure 6 shows the remaining concentration of H2O2 after its addition to the electrolyte solution. In all cases, approximately 50% of the initial H2O2 concentration has been consumed after 5 min; a further gradual consumption is observed. After 120 min, the concentration of H2O2 remains at 6.5 mM for cases of 0.2 and 0.3 M and 2 mM in case of 0.05 M initial H2O2 concentration. The large extent of removal of Cartap and the rapid consumption of H2O2 during the initial 5 min suggests that •OH generated according to Eq. (2) or at the BDD anode plays the main role in Cartap degradation. At this point, contribution of the direct oxidation of Cartap at the BDD anode cannot be estimated. As the result of rapid decomposition of Cartap, the pH value of solution (see insert of Fig. 6) decreases rapidly from 3 to 2.2 after 5 min and does not change thereafter. It can be assumed that the formation of organic acids resulting from the degradation of Cartap reduces the pH of the treated solution.
The TOC reduction in the electro-Fenton process and the effect of pretreatment with 15 mM NaOCl are presented in Fig. 7.
It is surprising that at lowest H2O2 concentration TOC decreases approximately to 42% after 5 min and then remains constant whereas TOC decreases for the cases of 0.2 M and 0.3 M H2O2 are very similar, only reaching to below 70% after 5 min. However, TOC for all cases decreases rapidly for 30 min after the start of the experiment and decreases slowly to 22% and 18% for 0.2 and 0.3 M H2O2, respectively, at the end of the process. It can be concluded that a lower concentration of H2O2 (0.05 M) results in higher efficiency of TOC removal than higher ones (0.2 and 0.3 M) in the initial of 5 min. This may be due to the possibility that excess H2O2 at the early stage (5 min) can also scavenge •OH to produce less oxidative species like ●OOH (Eq. (6)) [14] or can undergo competing reactions (Eqs. (7–8)) [41, 42] which result in the loss of oxidative capability.
This may also explain why the highest concentration of H2O2 (0.3 M) does not cause much higher TOC removal compared with the lower concentration (0.2 M).
The results of an investigation whether pretreatment of solution with NaOCl has positive impact on the electro-Fenton process are also depicted in Fig. 7. Although the TOC value remains at 78% after treatment with 15 mM NaOCl, further treatment by the electro-Fenton process does not result in further degradation. Possibly, the presence of Cl− ions formed from OCl− during oxidative decomposition of organics (Eq. 9)
has a negative impact on the Fenton process and its variation photo-Fenton process [43,44,45]. It may become evident by decreased rates of degradation and mineralization, formation of chlorinated compounds, etc. The negative impact of chloride ions on the Fenton process itself may be envisaged in various possible ways [43]: (i) the complexation of Fe2+/Fe3+ with Cl− (Eqs. 10–13) [46] inhibiting participation of Fe2+/Fe3+ ions in the Fenton reaction itself and later the electrochemical regeneration of Fe2+ by electroreduction of Fe3+ and (ii) scavenging of hydroxyl radicals (Eq. 14) [47] resulting in the generation of chlorine radicals Cl• (Eq. (15)) which are less reactive than the hydroxyl radical and may decompose without participating in the desired oxidation reaction (Eq. 16).
Effects of metal ion catalysts
It is well known that the Fenton reaction is catalyzed by several metal ions [14]. In order to investigate the influence of metal ions on the electro-Fenton process, degradation was investigated in the presence of three different metal ions: Mg2+, Al3+, and Cu2+. The initial metal ion concentration was 5 mM.
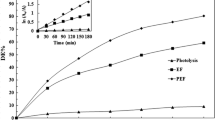
As shown in Fig. 8, the electro-Fenton process is effective up to 60 min. Although Al3+ or Mg2+ as co-catalysts slow down the process within the interval time 5 to 60 min, more than 80% TOC removal can be achieved after 120 min.
Figure 8 shows that adding 5 mM Cu2+ can accelerate TOC removal in the initial 30 min and only 35% of TOC remain in the treated solution after 5 min already. Further treatment can reduce the TOC value only to 22%. Higher activity of Cu(II) than Fe(II) has been noticed by Chumakov [33].
This positive effect is possibly due to the fast destruction of complexes of Cu2+ with carboxylic acids [26, 30] and the enhanced generation of •OH from the redox couple Cu2+/Cu+ (Eq. 19) [26, 48] in which Cu+ is formed from Cu2+ with HO2● (Eq. 18) (weaker oxidants produced in the electrolytic system), followed by regeneration of Cu2+ by oxidation of Cu+ with H2O2 from the Fenton-like reaction (19) [49]:
It can be concluded that although co-catalysts have some impact (positive/negative) at the early stages (less than 60 min), TOC/TOC0 in all cases is the same at around 20% at the end of the process. Compared with the reactivity rating established by Strlič et al., we also identify Cu2+ ions as providing the highest acceleration of the process.
Effect of Fe2+ concentration
According to several studies, the dosage of Fe2+ plays an important role on the efficiency of the electro-Fenton process [50,51,52,53]. As shown in Fig. 9, the rate of TOC decreases is accelerated with increasing Fe2+ concentration.
Meanwhile TOC decreases continuously during 120 min in the case of 5 mM Fe2+, there is no considerable reduction in TOC for the cases of 10 mM and 15 mM after 60 min. However, in all cases, the same value of TOC at approximately 20% is reached after 120 min of treatment time. This can be explained by assuming that in the early stages (less than 60 min) the higher Fe2+ concentration promotes production of •OH radical leading to faster decrease of the TOC value. Further treatment time does not contribute remarkably to TOC decay. This may be due to the formation of recalcitrant products that are difficult to be destroyed by BDD (•OH) and/or the Fenton reagent (•OH).
On the other hand, several previous studies [54,55,56] have pointed out that the use of excess Fe2+ influences the process negatively, which is explained in Eqs. 20–22 [14]:
Thus, using high concentration of Fe2+ should be considered, because the ferrous ions not only generate hydroxyl radicals but also are scavengers of •OH.
Effect of pH
The pH of the solution is a very important parameter in the Fenton process [57]. It affects directly the mechanism of oxidation, because a change in pH of the solution involves a variation of the concentration of Fe2+, and therefore, the formation rate of •OH radicals is affected. At high pH (pH > 4), the generation of •OH becomes slower due to the formation of various iron oxide/hydroxide species; iron complexes would further form \( \mathrm{Fe}{\left(\mathrm{OH}\right)}_4^{-} \) when the pH in the treated solution is higher than 9.0 [58].
On the other hand, at very low pH values (< 2.0), the reaction is slowed down by the formation of complex species [Fe(H2O)6]2+, which react more slowly with peroxide as compared to [Fe(OH)(H2O)5]2+. In addition, the peroxide gets solvated in the presence of high concentrations of H+ ions to form stable oxonium ions [H3O2]+ [59]. The oxonium ion makes peroxide more electrophilic enhancing its stability and presumably reduces substantially the reactivity with Fe2+ ions [60, 61].
Many previous studies [41, 49, 59] have pointed out that pH = 3 is the optimal value for the Fenton reaction. Thus, we have run a treatment for comparison with initial pH = 3; this value was maintained throughout the treatment.
The influence of pH on TOC removal is depicted in Fig. 10. As seen in insert of Fig. 6, pH of solution remains at 2.1 after 5 min. With initial pH = 3, the TOC value (see Fig. 10) gradually decreases by 23% and there is no significant change of the TOC value after 60 min. It is surprising that keeping the pH of the solution at 3 results in only further 5% decay of TOC (from 75% at 5 min to 70% at 120 min).
In previous studies, pH values in this range from 2 to 3 were maintained. For example, in the study of El-Ghenymy et al. [62], TOC decreases are very similar for pH = 2 and 3 at 83 and 85% of TOC removed, respectively. The same result can also be found elsewhere [49] with no difference in TOC decrease between pH = 2 and 3. On the other hand, in the study [10], the initial TOC value decreased by 90% at pH = 3 but only by 50% at pH = 2 during 120 min.
It can be concluded that any addition of OH− ions to reach the initial value pH = 3 of solution at the start of the process will slow down the TOC decay. It can be assumed that the added OH− ions are not consumed for changing the pH value of solution but are initially consumed for decomposing H2O2 or oxidizing Fe2+ and precipitating Fe(OH)3 as described in Eq. 23.
Obviously, pH adjustment by addition of OH− ions during the Fenton process is not useful.
Identification of intermediates and proposed degradation pathways
Degradation pathways for Cartap with the original Fenton process have been proposed elsewhere [5]. Figure 11 depicts HPLC chromatograms recorded with samples taken at various time intervals during the degradation (120 min in total) of Cartap. Padan 95SP contains 5% additives, but it is assumed that their presence does not interfere significantly with the determination of the intermediates from degradation of Cartap.
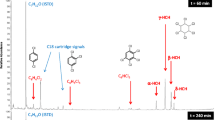
As can be seen in Fig. 11, Cartap (tr = 3.1 min) is decomposed almost completely within the initial 5 min of the electro-Fenton process. Accordingly, the efficiency of the process applied here is much higher than that of the original Fenton process examined earlier [5]. New peaks appear and then fade with reaction time. All peaks are present at electrolysis times less than 5 min, and their retention times are close to each other. Therefore, it is difficult to single out a main peak characteristic of just one intermediate or product. However, based on previous studies [5] and conceivable reaction pathways of related organic compounds with composition and structure similar to Cartap as proposed elsewhere [63,64,65,66,67], a degradation pathway of Cartap in the electro-Fenton process is suggested in Fig. 12.
Because Cartap is an ionic compound, deprotonation and release of HCl into the solution may proceed initially, consequently Cartap changes into compound A in the solution [5]. The alkane chain is degraded into short-chain organic acids [5]. The amino group at the end of the Cartap chain is oxidized into a nitro group yielding product B. The attack of •OH can also break the N–C bond in the main chain releasing smaller molecules (products C and D). This has been seen in previous studies [66, 67]. Compound D can be subsequently oxidized into E, F, or G, and finally into gaseous CO2 and inorganic ions (\( {\mathrm{NO}}_3^{-} \) and \( {\mathrm{NH}}_4^{+} \)). The C–S bond in compound C is broken to form product J and release SO2, CO2, H2O2, and \( {\mathrm{SO}}_4^{2-} \). In addition, product B can also subsequently be converted into product H to release NO and then the C–S chemical bond from H is destroyed for producing sulfuric acid in solution. Product I subsequently looses the amino group to form malonic acid. According to previous studies [66, 67], malonic acid is decomposed into acetic acid and formic acid.
It should be mentioned that the final degradation products are mineral ions such as \( {\mathrm{NH}}_4^{+} \), \( {\mathrm{SO}}_4^{2-},\mathrm{and}\ {\mathrm{NO}}_3^{-} \), resulting from the substituent groups of the initial molecule, as well as CO2 and H2O [70,71,72,73].
Identification of reaction intermediates was further attempted without success using GC-MS by examining small samples from the electrolyte solution taken at the same time intervals where samples for HPLC were taken. However, the volatilities of some intermediates may be too small for detection with GC-MS; some compounds cannot be observed with GC-MS due to their significant polarity [64].
As a further option, GC analysis with a flame ionization detector FID of the electro-Fenton process solution shows the formation of several intermediate products (see Fig. 13).
Conclusions
Pretreatment with 15 mM NaOCl can effectively remove Cartap in 700 mg L−1 Padan 95SP down to 22% of TOC at very short reaction time due to active chlorine, but it has a negative impact on the electro-Fenton process because of the remaining of Cl− ions in solution. The concentration of H2O2 and the dosage of Fe2+ are considered as the main parameters in the electro-Fenton process. However, the excess of both of them does not lead to increase in TOC removal. Using Cu2+ can enhance the TOC removal at the early stages but does not increase the effectiveness of process after 120 min of treatment. This limited effectiveness is due to the refractory by-products which cannot be decomposed by the attack of further •OH radicals.
This study also shows that the decrease of pH from 3 to 2.1 is the result of released organic acids; keeping the pH at 3 does not remarkably increase TOC removal. At the optimal conditions ([Padan 95SP]0 = 700 mg L−1, Vsolution = 250 mL, j = 20 mA cm−2, [Na2SO4] = 0.05 M, pH = 3, [H2O2]0 = 0.2 M, [Fe2+] = 10 mM), 20% of TOC still remained in solution. A reaction pathway of Cartap mineralization is proposed based on previous studies as well as our observations.
Notes
The presence of Cartap and its hydrochloride depends on the pH of the aqueous environment. A study of a conceivable distribution between both forms was not the subject of this study; thus, we use simply the term Cartap in the following text.
As a subfield, electrochemical AOPs (EAOPs) have been specified [9].
References
Boorugu HK, Chrispal A (2012) Cartap hydrochloride poisoning: A clinical experience. Ind J Crit Care Med 16:58–59
https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/124.htm (accessed 13.02.2020)
Nagawa Y, Saji Y, Chiba S, Yui T (1971) Neuromuscular Blocking Actions of Nereistoxin and Its Derivatives and Antagonism by Sulfhydryl Compounds. Jpn J Pharmacol 21:185–197
Berg H (2001) Pesticide use in rice and rice-fish farms in the Mekong Delta, Vietnam. Crop Prot 20:897–905
Tian K, Ming C, Dai Y, Ake KMH (2015) Fenton degradation of Cartap hydrochloride: identification of the main intermediates and the degradation pathway. Water Sci Technol 72:1198–1205
Choi E, Cho IH, Park J (2004) The Effect of Operational Parameters on the Photocatalytic Degradation of Pesticide. J Environ Sci Health B 39:53–64
Wu M, Deng H, Fan Y, Hu Y, Guo Y, Xie L (2018) Rapid colorimetric detection of cartap residues by AgNP sensor with magnetic molecularly imprinted microspheres as recognition elements. Molecules 23:1443
Lee SJ, Caboni P, Tomizawa M, Casida JE (2004) Cartap Hydrolysis Relative to Its Action at the Insect Nicotinic Channel. J Agric Food Chem 52:95–98
Sires I, Brillas E, Oturan MA, Rodrigo MA, Panizza M (2014) Electrochemical advanced oxidation processes: today and tomorrow: A review. Environ Sci Pollut Res 21:8336–8367
Brillas E (2014) Electro-Fenton, UVA Photoelectro-Fenton and Solar Photoelectro-Fenton Treatments of Organics in Waters Using a Boron-Doped Diamond Anode: A Review. J Mex Chem Soc 58:239–255
Moreira FC, Boaventura RAR, Brillas E, Vilar VJP (2017) Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl Catal B-Environ 202:217–261
Brillas E, Arias C, Cabot PL, Centellas F, Garrido JA, Rodriguez RM (2006) Degradation of Organic Contaminants by Advanced Electrochemical Oxidation Methods. Port Acta 24:159–189
Botte GG (2017) Electrochemical technologies for water treatment, management, and efficiency. Interface 26(2):53–62
Walling C (1975) Fenton’s reagent revisited. Acc Chem Res 8:125–131
Strlič M, Kolar J, Šelih V-S, Kočar D, Pihlar B (2003) A comparative study of several transition metals in Fenton-like reaction systems at circum-neutral pH. Acta Chim Slov 50:619–632
Boye B, Dieng MM, Brillas E (2002) Degradation of Herbicide 4-Chlorophenoxyacetic Acid by Advanced Electrochemical Oxidation Methods. Environ Sci Technol 36:3030–3035
Kurt U, Apaydin O, Gonullu MT (2007) Reduction of COD in wastewater from an organized tannery industrial region by Electro-Fenton process. J Hazard Mater 143:33–40
Pignatello JJ, Oliveros E, MacKay A (2006) Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit Rev Environ Sci Technol 36:1–84
Nidheesh PV, Gandhimathi R (2012) Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination 299:1–15
Wang N, Zheng T, Zhang G, Wang P (2016) A review on Fenton-like processes for organic wastewater treatment. J Environ Chem Eng 4:762–787
Oonnittan A, Sillanpaa MET (2012) Water Treatment by Electro-Fenton Process. Curr Org Chem 16:2060–2072
He H, Zhou Z (2017) Electro-Fenton process for water and wastewater treatment. Crit Rev Environ Sci Technol 47:2100–2131
Ventura A, Jacquet G, Bermond A, Camel V (2002) Electrochemical generation of the Fenton’s reagent: application to atrazine degradation. Water Res 36:3517–3522
Gozmen B, Oturan MA, Oturan N (2003) Erbatur O Indirect Electrochemical Treatment of Bisphenol A in Water via Electrochemically Generated Fenton's Reagent. Environ Sci Technol 37:3716–3723
Guivarch E, Oturan N, Oturan MA (2003) Removal of organophosphurus pesticides from water by electrogenerated Fenton’s reagent. Environ Chem Lett 1:165–168
Brillas E, Banos MA, Camps S, Arias C, Cabot PL, Garrido JA, Rodríguez RM (2004) Catalytic effect of Fe2+, Cu2+ and UVA light on the electrochemical degradation of nitrobenzene using an oxygen-diffusion cathode. New J Chem 28:314–322
Da Pozzo A, Merli C, Sirés I, Garrido JA, Rodríguez RM, Brillas E (2005) Removal of the herbicide amitrole from water by anodic oxidation and electro-Fenton. Environ Chem Lett 3:7–11
Hanna K, Chiron S, Oturan MA (2005) Coupling enhanced water solubilization with cyclodextrin to indirect electrochemical treatment for pentachlorophenol contaminated soil remediation. Water Res 39:2763–2773
Wang A, Qu J, Ru J, Liu H, Ge J (2005) Mineralization of an azo dye Acid Red 14 by electro-Fenton’s reagent using an activated carbon fiber cathode. Dyes Pigments 65:227–233
Sirés I, Garrido JA, Rodríguez RM, Cabot PL, Centellas F, Arias C, Brillas E (2006) Electrochemical degradation of paracetamol from water by catalytic action of Fe2+, Cu2+ and UVA light on electrogenerated hydrogen peroxide. J Electrochem Soc 153:D1–D9
Irmak S, Yavuz HI, Erbatur O (2006) Degradation of 4-chloro-2-methylphenol in aqueous solution by electro-Fenton and photo-electro-Fenton processes. Appl Catal B-Environ 63:243–248
Xie YB, Li XZ (2006) Interactive oxidation of photoelectrocatalysis and electro-Fenton for azo dye degradation using TiO2-Ti mesh and reticulated vitreous carbon electrodes. J Mater Chem Phys 95:39–50
Chumakov A, Batalova V, Slizhov Y (2016) Electro-Fenton-like reactions of transition metal ions with electrogenerated hydrogen peroxide. AIP Conf Proc 1772:040004
Brillas E, Boye B, Sirés I, Garrido JA, Rodríguez RM, Arias C, Cabot PL, Comninellis C (2004) Electrochemical destruction of chlorophenoxy herbicides by anodic oxidation and electro-Fenton using a boron-doped diamond electrode. Electrochim Acta 49:4487–4496
Hoang NT (2019) PhD-thesis Technische Universität Chemnitz available online at: https://nbn-resolving.org/urn:nbn:de:bsz:ch1-qucosa2-359516
Determination of cartap hydrochloride content (spectrophotometric method); Bureau of Indian Standards, IS 14159:1994
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Riddles PW, Blakeley RL, Zerner B (1983) Reassessment of Ellman’s reagent. Methods Enzymol 91:49–60
Eisenberg GM (1943) Colorimetric determination of hydrogen peroxide. Ind Eng Chem 15:327–328
Gottardi W, Bock V (1993) Direct iodometric determination of halogen (1+) compounds using arsenite. Fresenius J Anal Chem 347:400–408
El Haddad M, Regti A, Laamari MR, Mamouni R, Saffaj N (2014) Use of Fenton reagent as advanced oxidative process for removing textile dyes from aqueous solutions. J Mater Environ Sci 5:667–674
Chan KH, Chu W (2003) The dose and ratio effects of Fe(II) and H2O2 in Fenton’s process on the removal of atrazine. Environ Technol 24:703–710
Bacardit J, Stötzner J, Chamarro E, Esplugas S (2007) Effect of Salinity on the Photo-Fenton Process. Ind Eng Chem Res 46:7615–7619
Gaca J, Kowalska M, Mróz M (2005) The Effect of Chloride Ions on Alkylbenzenesulfonate Degradation in the Fenton Reagent. Pol J Environ Stud 14:23–27
Malik PK, Saha SK (2003) Oxidation of direct dyes with hydrogen peroxide using ferrous ion as catalyst. Sep Purif Technol 31:241–250
Lu M, Chang Y, Chen I, Huang Y (2005) Effect of chloride ions on the oxidation of aniline by Fenton’s reagent. J Environ Manag 75:177–182
Laat JD, Le TG, Legube B (2004) A comparative study of the effluents of chloride, sulphate and nitrate ions on the rates of decomposition of H2O2 and organic compounds by Fe(II)/H2O2 and Fe- (III)/H2O2. Chemosphere 55:715–723
Gallard H, De Laat J, Legube B (1999) Comparative study of the rate of decomposition of H2O2 and of atrazine by Fe(III)/H2O2, Cu(II)/H2O2, Fe(III)/Cu(II)/H2O2. Rev Sci Eau 12:715–728
Flox C, Ammar S, Arias C, Brillas E, Vargas-Zavala AV, Abdelhedi R (2006) Electro-Fenton and photoelectro-Fenton degradation of indigo carmine inacidic aqueous medium. Appl Catal B-Environ 67:93–104
Mansour D, Fourcade F, Bellakhal N, Dachraoui M, Hauchard D, Amrane A (2012) Biodegradability Improvement of Sulfamethazine Solutions by Means of an Electro-Fenton Process. Water Air Soil Pollut 223:2023–2034
Abdessalem AK, Bellakhal N, Oturan N, Dachraoui M, Oturan MA (2010) Treatment of a Mixture of Three Pesticides by Photo and Electro-Fenton Processes. Desalination 250:450–455
Oturan MA, Edelahi MC, Oturan N, El Kacemi K, Aaron J (2010) Kinetics of Oxidative Degradation/Mineralization Pathways of the Phenylurea Herbicides Diuron, Monuron and Fenuron in Water during Application of the Electro-Fenton Process. Appl Catal B-Environ 97:82–89
Hammami S, Oturan N, Bellakhal N, Dachraoui M, Oturan MA (2007) Oxidative Degradation of Direct Orange 61 by Electro-Fenton Process Using a Carbon Felt Electrode: Application of the Experimental Design Methodology. J Electroanal Chem 610:75–84
Karatas M, Argun YA, Argun ME (2012) Decolorization of antraquinonic dye, Reactive Blue 114 from synthetic wastewater by Fenton process: Kinetics and thermodynamics. J Ind Eng Chem 18:1058–1062
Tang XY, Shuang XS, Hua LZ (2011) Degradation of polyvinyl-alcohol wastewater by Fenton’s reagent: Condition optimization and enhanced biodegradability. J Cent S Univ Technol 18:96–100
Wang C, Zhang S, Zhang Z, Zeng M, Yuji S (2014) Optimization and Interpretation of Fenton and UV/Fenton Processes for Degradation of Syringyl Lignin. J Environ Anal Chem 1:1000115
Kremer ML (2003) The Fenton reaction. Dependence of the rate on pH. J Phys Chem A 107:1734–1741
Kuo WG (1992) Decolorizing dye wastewater with Fenton’s reagent. Water Res 26:881–886
Gulkaya I, Surucu GA, Dilek FB (2006) Importance of H2O2/Fe2+ ratio in Fenton’s treatment of a carpet dyeing wastewater. J Hazard Mater B 136:763–769
Kwon BG, Lee DS, Kang N, Yoon J (1999) Characteristics of p-chlorophenol oxidation by Fenton’s reagent. Water Res 33:2110–2118
Schriman JP, Delavarenne SY (1979) Hydrogen Peroxide in Organic Chemistry. Edition et documentation industrielle, Paris
El-Ghenymy A, Garrido JA, Centellas F, Arias C, Cabot PL, Rodríguez RM, Brillas E (2012) Electro-Fenton and Photoelectro-Fenton Degradation of Sulfanilic Acid Using a Boron-Doped Diamond Anode and an Air Diffusion Cathode. J Phys Chem A 116:3404–3412
Kaneco S, Li N, Itoh KK, Katsumata H, Suzuki T, Ohta K (2009) Titanium dioxide mediated solar photocatalytic degradation of thiram in aqueous solution: kinetics and mineralization. Chem Eng J 148:50–56
Fan C, Tsui L, Liao MC (2011) Parathion degradation and its intermediate formation by Fenton process in neutral environment. Chemosphere 82:229–236
Brillas E, Mur E, Sauleda R, Sanchez L, Peral J, Domènech X, Casado J (1998) Aniline mineralization by AOPs: anodic oxidation, photocatalysis, electro-Fenton and photoelectro-Fenton processes. Appl Catal B-Environ 16:31–42
Sakkas VA, Albanis TA (2003) Photocatalyzed degradation of the biocides chlorothalonil and dichlofluanid over aqueous TiO2 suspensions. Appl Catal B-Environ 46:175–188
Brillas E (2014) A review on the degradation of Organic Pollutants in Waters by UV Photoelectro-Fenton and Solar Photoelectro-Fenton. J Braz Chem Soc 25:393–417
Duan X, Zhao Y, Liu W, Chang L (2014) Investigation on electro-catalytic oxidation properties of carbon nanotube-Ce-modified PbO2 electrode and its application for degradation of m-nitrophenol. Arab J Chem. https://doi.org/10.1016/j.arabjc.2014.11.025
Duan X, Zhao Y, Liu W, Chang L, Li X (2014) Electrochemical degradation of p-nitrophenol on carbon nanotube and Ce-modified-PbO2 electrode. J Taiwan Inst Chem Eng 45:2975–2985
Maletzky P, Bauer R (1998) The photo-Fenton method degradation of nitrogen containing organic compounds. Chemosphere 37:899–909
Tanaka K, Padermole K, Hisanaga T (2000) Photocatalytic degradation of commercial azo dyes. Water Res 34:327–333
Joseph J, Destaillats H, Hung H, Hoffman M (2000) The sonochemical degradation of azobenzene and related azo dyes: rates enhancements via Fenton’s reactions. J Phys Chem A104:301–307
Guivarch E, Trevin S, Lahitte C, Oturan MA (2003) Degradation of azo dyes in water by Electro-Fenton process. Environ Chem Lett 1:38–44
Acknowledgments
This study was supported by the Vietnam International Education Development and Chemnitz University of Technology (Germany). We wish to express our thanks to Dr. E. Dietzsch, M. Hofmann, and Prof. Dr. M. Mehring (Chemnnitz University of Technology) as well as Nguyen Dinh Chuong (Department of BIN Convergence Technology, Chonbuk National University, Republic of Korea) and Vo Thang Nguyen (Analytical and Environmental Chemistry, Department of Chemistry, Danang University of Education) for experimental support and helpful discussions.
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Jose Zagal on the occasion of his 70th birthday in recognition of his numerous contributions to fundamental and applied electrochemistry.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hoang, N.T., Holze, R. Degradation of pesticide Cartap in Padan 95SP by combined advanced oxidation and electro-Fenton process. J Solid State Electrochem 25, 73–84 (2021). https://doi.org/10.1007/s10008-020-04581-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04581-7