Abstract
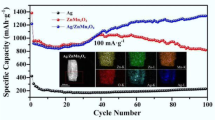
In the present work, the poly(o-phenylenediamine)/Ag (PoPD/Ag) hybrid composite with the microrod morphology was prepared by in situ chemical oxidation method, and then composite electrodes of PoPD/Ag (PVDF) and PoPD/Ag (sodium alginate) were prepared by using PVDF and sodium alginate as electrode binders, respectively. As explored as the anode of lithium ion batteries, the redox-active PoPD in the hybrid composite, compared to the pure PoPD, demonstrated the remarkably improved electrochemical performances with superior high capacity and good rate capability and cycling stability, due to the improved intrinsic electrical conductivity of PoPD via the introduced Ag nanoparticles in the polymer. As the sodium alginate was chosen as the functional electrode binder, PoPD/Ag (sodium alginate) exhibited the initial charge/discharge-specific capacity of 1452.9/2317.7 mAh g−1, and after the 100th cycles, the developed discharge capacity for PoPD/Ag (sodium alginate) reached to 1389.9 mAh g−1, which was evidently higher than that of PoPD. Its stable and representative discharge-specific capacities were 1291, 1044.9, 856.7, 762.5 mAh g−1 at the current rate of 50, 100, 200, and 500 mAh g−1, respectively, which were obviously superior to that of PoPD and PoPD/Ag (PVDF), indicating that sodium alginate as binder has the special effects on the electrochemical characteristics of PoPD, which benefited to the capacity release step by step during the charge/discharge process. The works would provide significant exploration for the preparation of high-performance poly (aromatic amine) polymer as the electrode materials.












Similar content being viewed by others
References
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367
Armand M, Tarascon JM (2008) Building better batteries. Nature 451:652–657
Cheng F, Liang J, Tao Z, Chen J (2011) Functional materials for rechargeable batteries. Adv Mater 23:1695–1715
Liang Y, Tao Z, Chen J (2012) Organic electrode materials for rechargeable lithium batteries. Adv Energy Mater 2:742–769
Song Z, Zhou H (2013) Towards sustainable and versatile energy storage devices: an overview of organic electrode materials. Energy Environ Sci 6:2280–2301
Oyama N, Pope JM, Sotomura T (1997) Effects of adding copper(II) salt to organosulfur cathodes for rechargeable lithium batteries. J Electrochem Soc 144:L47–L51
Oyaizu K, Nishide H (2009) Radical polymers for organic electronics: a radical departure from conjugated polymers? Adv Mater 21:2339–2344
Janoschka T, Hager MD, Schubert US (2012) Powering up the future: radical polymers for battery applications. Adv Mater 24:6397–6409
Oyama N, Tatsuma T, Sato T, Sotomura T (1995) Dimercaptan-polyaniline composite electrodes for lithium batteries with high energy density. Nature 373:598–600
Zhan L, Song Z, Zhang J, Tang J, Zhan H, Zhou Y, Zhan C (2008) PEDOT: cathode active material with high specific capacity in novel electrolyte system. Electrochim Acta 53:8319–8323
Abouimrane A, Weng W, Eltayeb H, Cui Y, Niklas J, Poluektov O, Amine K (2012) Sodium insertion in carboxylate based materials and their application in 3.6 V full sodium cells. Energy Environ Sci 5:9632–9638
Huang W, Zhu Z, Wang L, Wang S, Li H, Tao Z, Shi J, Guan L, Chen J (2013) Quasi-solid-state rechargeable lithium-ion batteries with a calix[4]quinone cathode and gel polymer electrolyte. Angew Chem Int Ed 52:9162–9166
Luo W, Allen M, Raju V, Ji X (2014) An organic pigment as a high-performance cathode for sodium-ion batteries. Adv Energy Mater 4:1400554
Wang HG, Yuan S, Ma DL, Huang XL, Meng FL, Zhang XB (2014) Tailored aromatic carbonyl derivative polyimides long-cyclesodium-organic batteries. Adv Energy Mater 4:1301651
Zhu Z, Hong M, Guo D, Shi J, Tao Z, Chen J (2014) All-solid-state lithium organic battery with composite polymer electrolyte and pillar[5]quinone cathode. J Am Chem Soc 136:16461–16464
Peng C, Zhang S, Jewell D, Chen GZ (2011) Carbon nanotube and conducting polymer composites for supercapacitors. Prog Nat Sci 18:777–788
Snook GA, Kao P, Best AS (2011) Conducting-polymer-based supercapacitor devices and electrodes. J Power Sources 196:1–12
Sivakkumar SR, Saraswathi R (2004) Performance evaluation of poly(N-methylaniline) and polyisothianaphthene in charge-storage devices. J Power Sources 137:322–328
JaIdev RS (2012) Poly(p-phenylenediamine)/graphene nanocomposites for supercapacitor applications. J Mater Chem 22:18775–18783
Wu JS, Rui XH, Long GK, Chen WQ, Yan QY, Zhang QC (2015) Pushing up lithium storage through nanostructured polyazaacene analogues as anode. Angew Chem 127:7462–7466
Li XG, Huang MR, Duan W, Yang YL (2002) Novel multifunctional polymers from aromatic diamines by oxidative polymerizations. Chem Rev 102:2925–3030
Long Pham Q, Haldorai Y, Nguyen VH, Tuma D, Shim JJ (2011) Facile synthesis of poly(p-phenylenediamine)/MWCNT nanocomposites and characterization for investigation of structural effects of carbon nanotubes. Bull Mater Sci 34:37–43
Oyama N, Ohsaka T (1987) Electrochemical properties of the polymer films prepared by electrochemical polymerization of aromatic compounds with amino groups. Synth Met 18:375–380
Zhang K, Zhang LL, Zhao XS, Wu J (2010) Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chem Mater 22:1392–1401
Chen J, Liu Y, Minett AI, Lynam C, Wang J, Wallace GG (2007) Flexible, aligned carbon nanotube/conducting polymer electrodes for a lithium-ion battery. Chem Mater 19:3595–3597
Yola ML, Gupta VK, Eren T, Şen AE, Atar N (2014) A novel electro analytical nanosensor based on graphene oxide/silver nanoparticles for simultaneous determination of quercetin and morin. Electrochim Acta 120:204–211
Beytur M, Kardaş F, Akyıldırım O, Özkan A, Atar N (2018) A highly selective and sensitive voltammetric sensor with molecularly imprinted polymer based silver@gold nanoparticles/ionic liquid modified glassy carbon electrode for determination of ceftizoxime. J Mol Liq 251:212–217
Atar N, Eren T, Yola ML (2015) Ultrahigh capacity anode material for lithium ion battery based on rod gold nanoparticles decorated reduced graphene oxide. Thin Solid Films 590:156–162.
Eren T, Atar N, Yola ML, Karimi-Maleh H, Çolak AT, Olgun A (2015) Facile and green fabrication of silver nanoparticles on a polyoxometalate for Li-ion battery. Ionics 21:2193–2199
Atar N, Eren T, Yola ML, Wang S (2015) Fe@Ag nanoparticles decorated reduced graphene oxide as ultrahigh capacity anode material for lithium-ion battery. Ionics 21:3185–3192
Guo Y, Zhou J, Song Y, Zhang L (2009) An efficient and environmentally friendly method for the synthesis of celuulose carbamate by microwave heating. Macromol Rapid Commun 30(17):1504–1508
Huang MR, Peng QY, Li XG (2006) Rapid and effective adsorption of lead ions on fine poly(phenylenediamine) micropartiles. Chem Eur J 12:4341–4350
Vijayashree MN, Subramanyam SV, Samuelson AG (1992) A new organic conducting material derived from 1,4-diaminoanthraquinone. Macromolecules 25:2988–2990
Yano J (1995) Electrochemical and structural studies on soluble and conducting polymer fromo-phenylenediamine. J Polym Sci Part A: Polym Chem 33:2435–2441
Premasiri AH, Euler WB (1995) Syntheses and characterization of poly(aminophenazines). Macromol Chem Phys 196:3655–3666
El-Rahman HAA (1997) Preparation and characterization of conducting copolymer films from 5-aminoquinoline and aniline. J Appl Electrochem 27:1061–1068
Funding
This research was financially supported by the National Science Foundation of China (Grant No.51573099), the Natural Science Foundation of Liaoning Province, China (Grant No.2015020641, No.201602591); Support Plan for Innovative Talents in Colleges and Universities in Liaoning Province (LR2017034); Basic Research Project of Key Laboratory of Liaoning Provincial Education Department (LZ2016005); and Liaoning Provincial Department of Education Project (Grant No. LQ2017010).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Su, C., Han, B., Ma, J. et al. Effects of silver nanoparticle on electrochemical performances of poly(o-phenylenediamine)/Ag hybrid composite as anode of lithium-ion batteries. J Solid State Electrochem 24, 1007–1015 (2020). https://doi.org/10.1007/s10008-020-04580-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04580-8