Abstract
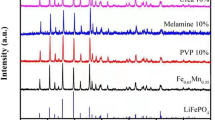
Polyaniline (PANI) was synthesized by adsorption double oxidizer method, and then reduced with N2H4·H2O (r-PANI), doped with HClO4 (d-PANI). PANI with different states was combined with lithium iron phosphate (LiFePO4) in water-based slurry to form PANI/LFP composites. Some physical characterization methods (Fourier transform infrared spectroscopy analysis, scanning electron microscope) were used to analyze the morphology and structure of the composites. The electrochemical properties were characterized by cyclic voltammetry, impedance spectroscopy, galvanostatic charge/discharge, and rate capability. The specific capacity of the r-PANI/LFP composite reached 154 mAh/g, which was 4.1% higher than that of pure LFP, and the coulombic efficiency of the battery was close to 100%. Under the higher discharge rate conditions, the r-PANI/LFP composite still released a good reversible capacity. Moreover, the cyclability and rate capability of the r-PANI/LFP composites are better than that of pure LFP.








Similar content being viewed by others
References
Padhi AK, Goodenough JB, Nanjundaswamy KS (1997) Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc 144:1188–1194
Arnold G, Garche J, Hemmer R, Ströbele S, Vogler C, Wohlfahrt-Mehrens M (2003) Fine particle lithium iron phosphate LiFePO4, synthesized by a new low-cost aqueous precipitation technique. J Power Sources 119:247–251
Vu A, Stein A (2011) Multiconstituent synthesis of LiFePO4/C composites with hierarchical porosity as cathode materials for lithium ion batteries. Chem Mater 23:3237–3245
Li CC, Chen CA, Chen MF (2017) Gelation mechanism of organic additives with LiFePO4, in the water-based cathode slurries. Ceram Int 43:765–770
Ozawa K (1994) Lithium-ion rechargeable batteries with LiCoO2, and carbon electrodes: the LiCoO2/C system. Solid State Ionics 69:212–221
Armstrong AR, Bruce PG (1996) Synthesis of layered LiMnO2 as an electrode for rechargeable lithium batteries. Nature 27:499–500
Ohzuku T, Ueda A, Nagayama M (1993) Electrochemistry and structural chemistry of LiNiO2 (R3̅m) for 4 volt secondary lithium cells. J Electrochem Soc 140:1862–1870
Yi TF, Li XY, Liu H, Shu J, Zhu YR, Zhu RS (2012) Recent developments in the doping and surface modification of LiFePO4, as cathode material for power lithium ion battery. Ionics 18:529–539
Iltchev N, Chen Y, Okada S, Yamaki J (2003) LiFePO4, storage at room and elevated temperatures. J Power Sources 119–121:749–754
Islam MS, Driscoll DJ, Fisher CAJ, Slater PR (2005) Atomic-scale investigation of defects, dopants, and lithium transport in the LiFePO4 olivine-type battery material. Chem Mater 17:5085–5092
Shin HC, Chung KY, Min WS, Byun DJ, Jang H, Cho BW (2008) asymmetry between charge and discharge during high rate cycling in LiFePO4, in situ X-ray diffraction study. Electrochem Commun 10:536–540
Wang H, Zhao N, Shi C, Ma L, He F, He C, Li J, Liu E (2017) Effect of interfacial lithium insertion on the stability and electronic structure of graphene/LiFePO4. Electrochim Acta 247:1030–1037
Chen M, Kou K, Tu M, Hu J, Du X, Yang B (2017) Conducting reduced graphene oxide wrapped LiFePO4/C nanocrystal as cathode material for high-rate lithium secondary batteries. Solid State Ionics 310:95–99
Chung SY, Blocking JT, Andersson AS, Chiang YM (2002) Electronically conductive phospho-olivines as lithium storage electrodes. Nature Mater 1:123–128
Gao L, Xu Z, Zhang S, Xu J, Tang K (2017) Enhanced electrochemical properties of LiFePO4 cathode materials by Co and Zr multi-doping. Solid State Ionics 305:52–56
Kang SH, Kim BR, Kim C, Park TJ, Son JT (2014) Electrochemical and morphologic studies of spherical LiFePO4/nanostructured LiFePO4, fibers composite by solid-state blending. Ceram Int 41:1963–1969
Eftekhari A (2017) LiFePO4/C Nanocomposites for lithium-ion batteries. J Power Sources 343:395–411
Huang YH, Park KS, Goodenough JB (2006) Improving lithium batteries by tethering carbon-coated LiFePO4 to polypyrrole. J Electrochem Soc 153:A2282–A2286
Huang YH, Goodenough JB (2008) High-rate LiFePO4 lithium rechargeable battery promoted by electrochemically active polymers. Chem Mater 20:7237–7241
Epstein AJ, Ginder JM, Zuo F, Bigelow RW, Woo H-S, Tanner DB, Richter AF, Huang W-S, MacDiarmid AG (1987) Insulator-to-metal transition in polyaniline. Synth Met 18:303–309
Wang R, Han M, Zhao Q, Ren Z, Guo X, Xu C, Hu N, Lu L (2017) Hydrothermal synthesis of nanostructured graphene/polyaniline composites as high-capacitance electrode materials for supercapacitors. Sci Rep 7:44562
Zhang LL, Zhao S, Tian XN, Zhao XS (2010) Layered graphene oxide nanostructures with sandwiched conducting polymers as supercapacitor electrodes. Langmuir the Acs Journal of Surfaces & Colloids 26:17624–17628
Singu BS, Male U, Srinivasan P, Yoon KR (2017) Preparation and performance of polyaniline–multiwall carbon nanotubes–titanium dioxide ternary composite electrode material for supercapacitors. J Ind Eng Chem 49:82–87
Ryu KS, Kim KM, Kang SG, Joo J, Chang SH (2000) Comparison of lithium/polyaniline secondary batteries with different dopants of HCl and lithium ionic salts. J Power Sources 88:197–201
Liu P, Han JJ, Jiang LF, Li ZY, Cheng JN (2017) Polyaniline/multi-walled carbon nanotubes composite with core-shell structures as a cathode material for rechargeable lithium-polymer cells. Appl Surf Sci 400:446–452
Chen WM, Long Q, Yuan LX, Xia SA, Hu XL, Zhang WX, Hung YH (2011) Insight into the improvement of rate capability and cyclability in LiFePO4/polyaniline composite cathode. Electrochim Acta 56:2689–2695
Chen WM, Huang YH, Yuan LX (2011) Self-assembly LiFePO4/polyaniline composite cathode materials with inorganic acids as dopants for lithium-ion batteries. J Electroanal Chem 660:108–113
Ait SA, Jozwiak P, Zaghib K, Garbarczyk J, Gendron F, Mauger A, Julien CM (2006) FTIR Features of lithium-iron phosphates as electrode materials for rechargeable lithium batteries. Spectrochim Acta Part A Mol Biomol Spectrosc 65:1007–1013
Ryu KS, Kim KM, Kang SG, Lee GJ, Joo J, Chang SH (2000) The charge/discharge mechanism of polyaniline films doped with LiBF4 as a polymer electrode in a Li secondary battery. Solid State Ionics 135:229–234
Ryu KS, Kim KM, Kang SG, Lee GJ, Joo J, Chang SH (2000) Electrochemical and physical characterization of lithium ionic salt doped polyaniline as a polymer electrode of lithium secondary battery. Synth Met 110:213–217
Acknowledgements
The authors are thankful for the support provided by School of Marine Science and Technology, Harbin Institute of Technology, Weihai.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Han, JJ., Guo, AR. & Wang, YF. Synthesis of PANI and its application in LiFePO4 cathode material. Ionics 28, 1073–1080 (2022). https://doi.org/10.1007/s11581-021-04414-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-021-04414-1