Abstract
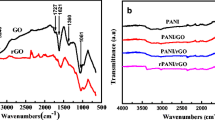
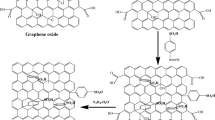
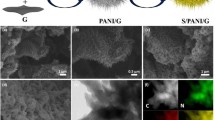
Polyaniline, polyaniline/graphene composites were synthesized by a novel in situ chemical oxidative polymerization method including two oxidants. The morphology and structure of the material were characterized by field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FT-IR). The electrochemical performance of polyaniline (PANI)-based composites was tested by electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV) testing, and constant current charge and discharge (GCD) tests. At 0.2 C of constant current, the discharge specific capacities of PANI/graphene oxide (PANI/GO) and PANI/GO-sodium borohydride (graphene oxide is reduced by sodium borohydride, named PANI/GO-NaBH4) were as high as 183 mAh/g and 192 mAh/g, respectively, which was nearly twice as high as that of PANI (100 mAh/g). After 100 charge and discharge cycles, the capacity retention rates of PANI, PANI/GO, and PANI/GO-NaBH4 were 80.4%, 89.4%, and 95.05%, respectively; the cycle performance was greatly improved before the modification. These results indicate that the composite has exciting potentials for the cathode material of zinc-rechargeable battery.












Similar content being viewed by others
References
Ciric-Marjanovic G (2013) Recent advances in polyaniline composites with metals, metalloids and nonmetals. Synth Met 170:31–56
Xia C, Guo J, Lei Y, Liang H, Zhao C, Alshareef (2017) Rechargeable aqueous zinc-ion battery based on porous framework zinc pyrovanadate intercalation cathode. Adv Mater 30: 1705580
Nuramdhani I, Gokceoren AT, Odhiambo SA, De MG, Hertleer C, Van LL (2018) Electrochemical impedance analysis of a pedot: pss-based textile energy storage device. Materials 11:48
Cheng C, Rui X, Shen W (2018) A lithium-ion battery-in-the-loop approach to test and validate multi-scale dual H infinity filters for state of charge and capacity estimation. IEEE Trans Power Electron PP:1–1
Wang H, Tao Z, Fu Y, Xiao H, Bai H (2019) Research on influencing factors for consistency performance of lithium ion batteries. IOP Conf Ser Earth Environ Sci 223:012–036
Nordrum A (2019) A safer way for batteries to fail: putting a gap in the right place can stop lithium-ion batteries from exploding. IEEE Spectr 56:12–13
Liu G, Yu Y, Jing H, Wei X, Liu X, Liu Y (2014) An ecological risk assessment of heavy metal pollution of the agricultural ecosystem near a lead-acid battery factory. Ecol Indic 47:210–218
Liu X, Cai JC, Shu YH (2014) The elimination of pollution of toxic cadmium and arsenic in lead-based alloys of lead-acid batteries in China. Adv Mater Res 983:319–323
Xu C, Li B, Du H, Kang F (2012) Energetic zinc ion chemistry: the rechargeable zinc ion battery. Angew Chem 51(4):933–935
Kundu D, Adams BD, Duffort V, Vajargah SH, Nazar LF (2016) A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat Energy 1:16119
Zhang L (2017) Mn3O4/carbon nanotube nanocomposites recycled from waste alkaline Zn–MnO2 batteries as high-performance energy materials. Rare Metals 36:442–448
Sun W (2017) Zn/MnO2 battery chemistry with H(+) and Zn(2+) coinsertion. J Am Chem Soc 139(29):9775–9778
Mones ES, Gillado AV, Herrera MU (2016) Photoresponse of zinc oxide-polyaniline junction at different light intensities. Key Eng Mater 705:4
Rafiqi FA, Majid K (2016) Synthesis, characterization, photophysical, thermal and electrical properties of composite of polyaniline with zinc bis (8-hydroxyquinolate): a potent composite for electronic and optoelectronic use. RSC Adv 6:22016–22025
Kan J, Xue H, Mu S (1998) Effect of inhibitors on Zn-dendrite formation for zinc-polyaniline secondary battery. J Power Sources 74:113–116
Huang J, Zhuo W, Hou M (2018) Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery. Nat Commun 9:2906
Wang X, Xin J, Dawei GU, Shen L (2006) A novel Zn-PANI dry rechargeable battery. Rare Metals 25:67–70
Mohsen RM, Morsi SMM, Selim MM, Ghoneim AM, El-Sherif HM (2018) Electrical, thermal, morphological, and antibacterial studies of synthesized polyaniline/zinc oxide nanocomposites. Polym Bull 76:1–21
Ghanbari K (2007) Synthesis of polyaniline/graphite composite as a cathode of Zn-polyaniline rechargeable battery. J Power Sources 170:513–519
Dizon JCM, Tapia AKG, Razado-Colambo I, Herrera MU (2018) Fabrication of polyaniline on silane-functionalized zinc oxide. Key Eng Mater 775:94–98
Liu P (2017) Polyaniline/multi-walled carbon nanotubes composite with core-shell structures as a cathode material for rechargeable lithium-polymer cells. Appl Surf Sci 400:446–452
Fonseca BL, Fonseca MA, Oliveira MSA (2018) Thermo-mechanical characterization of shape-memory polyurethane nanocomposites filled with carbon nanotubes and graphene nanosheets. Polym Compos 39:1216–1223
Chen C, Xi J, Zhou E, Li P, Chen Z, Chao G (2018) Porous graphene microflowers for high-performance microwave absorption. Nano-Micro Letters 10:26
Moreno C, Vilas-Varela M, Kretz B, Garcia-Lekue A, Costache MV, Paradinas M (2018) Bottom-up synthesis of multifunctional nanoporous graphene. Science 360:199–203
Gupta RK, Alahmed ZA, Yakuphanoglu F (2013) Graphene oxide based low cost battery. Mater Lett 112:75–77
Farooqui UR, Ahmad AL, Hamid NA (2018) Graphene oxide: a promising membrane material for fuel cells. Renew Sust Energ Rev 82:714–733
Dreyer DR, Park S, Bielawski CW (2010) The chemistry of graphene oxide. Chem Soc Rev 39(1):228–240
Weijie W, Jian Y, Jiaqin L, Dawei O, Qingqing Q, Binbin L (2018) Self-healing polyaniline-graphene oxides based electrodes with enhanced cycling stability. Electrochimica 282:835–844
Harfouche N, Gospodinova N, Nessark B, Perrin FX (2017) Electrodeposition of composite films of reduced graphene oxide/polyaniline in neutral aqueous solution on inert and oxidizable metal. J Electroanal Chem 786:135–144
Almeida DAL, Couto AB, Ferreira NG (2019) Flexible polyaniline/reduced graphene oxide/carbon fiber composites applied as electrodes for supercapacitors. J Alloys Compd 788:453–460
Saha S, Mitra M, Sarkar A, Banerjee D, Ganguly S, Kargupta K (2018) Lithium assisted enhanced hydrogenation of reduced graphene oxide-PANI nanocomposite at room temperature. Diam Relat Mater 84:103–111
Shi HY, Ye YJ, Liu K, Song Y, Sun X (2018) A long cycle-life self-doped polyaniline cathode for rechargeable aqueous zinc batteries. Angew Chem Int Ed 57(50):16359–16363
Ji C, Yao B, Li C, Shi G (2013) An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 64:225–229
Guerrero-Contreras J, Caballero-Briones F (2015) Graphene oxide powders with different oxidation degree, prepared by synthesis variations of the Hummers method. Mater Chem Phys 153:209–220
Zhang P, Han X, Kang L, Qiang R, Liu W, Du Y (2013) Synthesis and characterization of polyaniline nanoparticles with enhanced microwave absorption. RSC Adv 3:12694
Remyamol T, John H, Gopinath P (2013) Synthesis and nonlinear optical properties of reduced graphene oxide covalently functionalized with polyaniline. Carbon 59:308–314
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Z., Han, JJ., Zhang, N. et al. Synthesis of polyaniline/graphene composite and its application in zinc-rechargeable batteries. J Solid State Electrochem 23, 3373–3382 (2019). https://doi.org/10.1007/s10008-019-04435-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-019-04435-x