Abstract
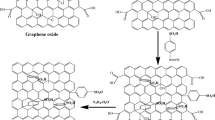
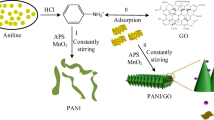
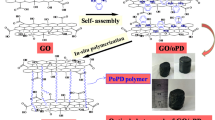
Graphene oxide (GO) was synthesized by an improved Hummers method and then reduced with NaBH4; GO became rGO with regular layered structure. Polyaniline (PANI)/rGO composite was prepared by a adsorption double oxidant method with rGO as a template. Some physical characterization methods (Fourier transform infrared spectroscopy analysis, X-ray diffraction, scanning electron microscope, and transmission electron microscope) were used to analyze the morphology and crystallinity of the composite. The electrochemical properties were characterized by cyclic voltammetry, impedance spectroscopy, galvanostatic charge/discharge, and rate capability. The first discharge specific capacity of the rPANI/rGO and PANI/rGO was 181.2 and 147.8 mAh/g. After 100 cycles, the capacity retention rate was still 90.2 and 88.9% separately, and the coulombic efficiency of batteries is close to 100%. These results demonstrate the composite has exciting potentials for the cathode material of lithium-ion battery.






Similar content being viewed by others
References
Shirakawa H, Louis EJ, Macdiarmid AG et al (1977) Synthesis of electrically conducting organic polymers: halogen derivatives of polyacetylene, (CH)x. Journal of the chemical society. Chem Commun 16(16):578–580
Wochnowski C, Metev S (2002) UV-laser-assisted synthesis of iodine-doped electrical conductive polythiophene. Appl Surf Sci 186(1–4):34–39
Qian R, Qiu J (1987) Electrochemically prepared polypyrroles from aqueous solutions. Polym J 18(1–3):13–18
Macdiarmid AG, Shao-Lin M, Somasiri NLD et al (1985) Electrochemical characteristics of “polyaniline” cathodes and anodes in aqueous electrolytes. Mol Cryst Liq Cryst 121(1–4):187–190
Macdiarmid AG, Chiang J-C, Halpern M, Huang W-S, Mu S-L, Nanaxakkara LD, Wu SW, Yaniger SI (1985) “Polyaniline”: interconversion of metallic and insulating forms. Mol Cryst Liq Cryst 121(1–4):173–180
Armelin E, Pla R, Liesa F, Ramis X, Iribarren JI, Alemán C (2008) Corrosion protection with polyaniline and polypyrrole as anticorrosive additives for epoxy paint. Corros Sci 50(3):721–728
Epstein AJ, Ginder JM, Zuo F, Bigelow RW, Woo HS, Tanner DB, Richter AF, Huang WS, MacDiarmid AG (1987) Insulator-to-metal transition in polyaniline. Synth Met 18(1):303–309
Watanabe A, Mori K, Iwasaki Y, Nakamura Y, Niizuma S (1987) Electrochromism of polyaniline film prepared by electrochemical polymerization. Macromolecules 20(8):1793–1796
Kalaivasan N, Shafi SS (2017) Enhancement of corrosion protection effect in mechanochemically synthesized polyaniline/MMT clay nanocomposites. Arab J Chem 10(2):S127–S133
Wang R, Han M, Zhao Q, Ren Z, Guo X, Xu C, Hu N, Lu L (2017) Hydrothermal synthesis of nanostructured graphene/polyaniline composites as high-capacitance electrode materials for supercapacitors. Sci Rep 7:44562
Singu BS, Male U, Srinivasan P et al (2017) Preparation and performance of polyaniline–multiwall carbon nanotubes–titanium dioxide ternary composite electrode material for supercapacitors. J Ind Eng Chem 49
Ryu KS, Kim KM, Kang SG, Joo J, Chang SH (2000) Comparison of lithium/polyaniline secondary batteries with different dopants of HCl and lithium ionic salts. J Power Sources 88(2):197–201
He BL, Dong B, Wang W, Li HL (2009) Performance of polyaniline/multi-walled carbon nanotubes composites as cathode for rechargeable lithium batteries. Mater Chem Phys 114(1):371–375
Liu P, Han JJ, Jiang LF, Li ZY, Cheng JN (2017) Polyaniline/multi-walled carbon nanotubes composite with core-shell structures as a cathode material for rechargeable lithium-polymer cells. Appl Surf Sci 400:446–452
Ritchie A, Howard W (2006) Recent developments and likely advances in lithium-ion batteries. J Power Sources 162(2):809–812
Fergus JW (2010) Recent developments in cathode materials for lithium ion batteries. J Power Sources 195(4):939–954
Ozawa K (1994) Lithium-ion rechargeable batteries with LiCoO2, and carbon electrodes: the LiCoO2/C system. Solid State Ionics 69(3–4):212–221
Graetz J, Hightower A, Ahn CC et al (2017) Electronic structure of chemically-delithiated LiCoO2 studied by electron energy-loss spectrometry. J Phys Chem B 106(6):1286–1289
Padhi AK, Goodenough JB, Nanjundaswamy KS (1997) Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc 144(4):1188–1194
Yi TF, Li XY, Liu H, Shu J, Zhu YR, Zhu RS (2012) Recent developments in the doping and surface modification of LiFePO4, as cathode material for power lithium ion battery. Ionics 18(6):529–539
Lee HW, Muralidharan P, Kim DK (2016) Synthesis of one-dimensional spinel LiMn2O4 nanostructures as a positive electrode in lithium ion battery. J Korean Ceram Soc 48(379):379–383
Cui P, Jia Z, Li L, He T (2011) Preparation and characteristics of Sb-doped LiNiO2, cathode materials for Li-ion batteries. J Phys Chem Solids 72(7):899–903
Gospodinova N, Terlemezyan L (1998) Conducting polymers prepared by oxidative polymerization: polyaniline. Prog Polym Sci 23(8):1443–1484
Ameen S, Shaheer Akhtar M, Husain M (2010) A review on synthesis processing, chemical and conduction properties of polyaniline and its nanocomposites. Sci Adv Mater 2(2):441–462
Liu Y, Zhou Y, Zhang S, Zhang J, Ren P, Qian C (2016) Amorphous iron phosphate/carbonized polyaniline nanorods composite as cathode material in sodium-ion batteries. J Solid State Electrochem 20(2):479–487
Zhou X, He T, Chen X et al (2017) Synthesis and lithium storage properties of vanadium oxide nanotubes (VOxNTs)-polyaniline nanocomposite as cathode material for lithium ion batteries. J Mater Sci Mater Electron:1–10
Dreyer DR, Park S, Bielawski CW et al (2014) The chemistry of graphene oxide. Chem Soc Rev 43(15):5288–5301
Zhu Y, Murali S, Cai W, Li X, Suk JW, Potts JR, Ruoff RS (2010) Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater 22(45):3906–3924
Compton OC, Nguyen ST (2010) Graphene oxide, highly reduced graphene oxide, and graphene: versatile building blocks for carbon-based materials. Small 6(6):711–723
Wang H, Hao Q, Yang X, Lu L, Wang X (2010) Effect of graphene oxide on the properties of its composite with polyaniline. ACS Appl Mater Interfaces 2(3):821–828
Gui D, Liu C, Chen F, Liu J (2014) Preparation of polyaniline/graphene oxide nanocomposite for the application of supercapacitor. Appl Surf Sci 307(8):172–177
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80(6):1339
Sun RK, Man KK, Seong-Gu K et al (2000) The charge/discharge mechanism of polyaniline films doped with LiBF4 as a polymer electrode in a Li secondary battery. Solid State Ionics 135:229–234
Ryu KS, Kim KM, Kang SG, Lee GJ, Joo J, Chang SH (2000) Electrochemical and physical characterization of lithium ionic salt doped polyaniline as a polymer electrode of lithium secondary battery. Synth Met 110(3):213–217
Funding
This study is the supported by the School of Marine Science and Technology, Harbin Institute of Technology, Weihai.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, RT., Han, JJ., Liu, P. et al. Synthesis of PANI/rGO composite as a cathode material for rechargeable lithium-polymer cells. Ionics 24, 3367–3373 (2018). https://doi.org/10.1007/s11581-018-2525-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2525-3