Abstract
Context
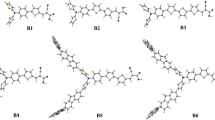
The organic solar cells (OSCs) are being developed with the goal of improving their photovoltaic capabilities. Here, utilizing computational methods, six new nonfullerene acceptors (NFA) comprising dyes (A1–A6) have been created by end-group alterations of the Y123 framework as a standard (R).
Methods
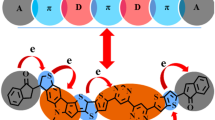
The DFT-based investigations at B3LYP/6-31G + (d,p) level were applied to evaluate their properties. The planar geometries associated with these structures, which lead to improved conjugation, were validated by the estimation of molecular geometries. Dyes A1–A6 have shorter Egap than R, according to a frontier molecular orbital (FMO) investigation, which encourages charge transfer in them. The dyes with their maximum absorption range were shown by optical properties to be 692–711 nm, which is significantly better than R with its 684 nm range. Their electrostatic and Mulliken charge patterns provided additional evidence of the significant separation of charges within these structures. All the dyes A1–A6 had improved light harvesting efficiency (LHE) values as compared to Y123, highlighting their improved capacity to generate charge carriers by light absorption. With the exception of dye A4, all newly developed dyes might have a superior rate of charge carrier mobility than R, according to reorganization energies λre. Dyes A3 and A4 had the greatest open-circuit voltage (Voc). Dye A3 exhibited improvement in all of its examined properties, making it a promising choice in DSSC applications.
Graphical Abstract














Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information file.
Code availability
Gaussian 09 W and GaussView 5.1 are used for simulation, and origin software is used to draw the plots.
References
Shahsavari A, Akbari M (2018) Potential of solar energy in developing countries for reducing energy-related emissions. Renew Sustain Energy Rev 90:275–291. https://doi.org/10.1016/j.rser.2018.03.065
Karakurt I, Aydin G (2023) Development of regression models to forecast the CO2 emissions from fossil fuels in the BRICS and MINT countries. Energy 263:125650. https://doi.org/10.1016/j.energy.2022.125650
Gereffi G, Dubay K, Robinson J, others (2008) Concentrating solar power clean energy for the electric grid by
Perez M, Perez R (2022) Update 2022–A fundamental look at supply side energy reserves for the planet. Sol Energy Adv 2:100014. https://doi.org/10.1016/j.seja.2022.100014
Green MA, Ho-Baillie A, Snaith HJ (2014) The emergence of perovskite solar cells. Nat Photonics 8:506–514. https://doi.org/10.1038/nphoton.2014.134
Kan B, Kan Y, Zuo L et al (2021) Recent progress on all-small molecule organic solar cells using small-molecule nonfullerene acceptors. InfoMat 3:175–200
Rand BP, Cheyns D, Vasseur K et al (2012) The impact of molecular orientation on the photovoltaic properties of a phthalocyanine/fullerene heterojunction. Adv Funct Mater 22:2987–2995
Li Y, Huang W, Zhao D et al (2022) Recent progress in organic solar cells: a review on materials from acceptor to donor. Molecules 27:1800. https://doi.org/10.3390/molecules27061800
Breitung EM, Shu C-F, McMahon RJ (2000) Thiazole and thiophene analogues of donor- acceptor stilbenes: molecular hyperpolarizabilities and structure- property relationships. J Am Chem Soc 122:1154–1160
Hagfeldt A, Boschloo G, Sun L et al (2010) Dye-sensitized solar cells. Chem Rev 110:6595–6663. https://doi.org/10.1021/cr900356p
Dey S (2019) Recent progress in molecular design of fused ring electron acceptors for organic solar cells. Small 15:1900134
Zheng Z, Yao H, Ye L et al (2020) PBDB-T and its derivatives: a family of polymer donors enables over 17% efficiency in organic photovoltaics. Mater Today 35:115–130. https://doi.org/10.1016/j.mattod.2019.10.023
Kang SB, Kim J-H, Jeong MH et al (2019) Stretchable and colorless freestanding microwire arrays for transparent solar cells with flexibility. Light Sci Appl 8:121. https://doi.org/10.1038/s41377-019-0234-y
Hu Z, Wang J, Ma X et al (2020) A critical review on semitransparent organic solar cells. Nano Energy 78:105376. https://doi.org/10.1016/j.nanoen.2020.105376
Horiuchi T, Miura H, Sumioka K, Uchida S (2004) High efficiency of dye-sensitized solar cells based on metal-free indoline dyes. J Am Chem Soc 126:12218–12219
Abe R, Shinmei K, Koumura N et al (2013) Visible-light-induced water splitting based on two-step photoexcitation between dye-sensitized layered niobate and tungsten oxide photocatalysts in the presence of a triiodide/iodide shuttle redox mediator. J Am Chem Soc 135:16872–16884. https://doi.org/10.1021/ja4048637
Kakiage K, Yamamura M, Fujimura E et al (2010) High performance of Si–O–Ti bonds for anchoring sensitizing dyes on TiO2 electrodes in dye-sensitized solar cells evidenced by using alkoxysilylazobenzenes. Chem Lett 39:260–262
Ramsdale CM, Barker JA, Arias AC et al (2002) The origin of the open-circuit voltage in polyfluorene-based photovoltaic devices. J Appl Phys 92:4266–4270
Hassan AU, Sumrra SH, Nkungli NK, Güleryüz C (2022) Theoretical probing of 3d nano metallic clusters as next generation non-linear optical materials. Results Chem 4:100627. https://doi.org/10.1016/j.rechem.2022.100627
Hassan AU, Sumrra SH, Mustafa G et al (2023) Molecular modeling of mordant black dye for future applications as visible light harvesting materials with anchors: design and excited state dynamics. J Mol Model 29:74. https://doi.org/10.1007/s00894-023-05474-y
Frisch JM, Trucks WG, Schlegel BH et al (2013) Gaussian 09, Revision D. 01, Gaussian, Inc. Wallingford, CT
Anthony JE (2011) Small-molecule, nonfullerene acceptors for polymer bulk heterojunction organic photovoltaics. Chem Mater 23:583–590
Sumrra SH, Hassan AU, Zafar MN et al (2022) Metal incorporated sulfonamides as promising multidrug targets: combined enzyme inhibitory, antimicrobial, antioxidant and theoretical exploration. J Mol Struct 1250:131710. https://doi.org/10.1016/j.molstruc.2021.131710
Li R, Liu J, Cai N et al (2010) Synchronously reduced surface states, charge recombination, and light absorption length for high-performance organic dye-sensitized solar cells. J Phys Chem B 114:4461–4464. https://doi.org/10.1021/jp101222s
Hassan AU, Sumrra SH, Mustafa G et al (2023) Creating intense and refined NLO responses by utilizing dual donor structural designs in A-π-D-π-D-π-A type organic switches: computed device parameters. Struct Chem. https://doi.org/10.1007/s11224-023-02138-8
Lonnecke K, Eberhardt O, Wallmersperger T (2023) Electrostatic charge distribution in armchair and zigzag carbon nanotubes: a numerical comparison of CNT charge models. Acta Mech 234:1–16. https://doi.org/10.1007/s00707-021-03085-3
Huang S, Shao W, Shi S et al (2022) The effect of conjugated groups for favourable molecular planarity and efficient suppression of charge recombination simultaneously of phenothiazine-based organic dyes for dye-sensitized solar cells. Synth Met 290:117137. https://doi.org/10.1016/j.synthmet.2022.117137
Ye J-T, Wang H-Q, Zhang Y, Qiu Y-Q (2019) Regulation of the molecular architectures on second-order nonlinear optical response and thermally activated delayed fluorescence property: homoconjugation and twisted donor–acceptor. J Phys Chem C 124:921–931
Kaur S, Mohiuddin G, Punjani V et al (2019) Structural organization and molecular self-assembly of a new class of polar and non-polar four-ring based bent-core molecules. J Mol Liq 295:111687
Aydın G, Koçak O, Güleryüz C, Yavuz I (2020) Structural order and charge transfer in highly strained carbon nanobelts. New J Chem 44:15769–15775. https://doi.org/10.1039/D0NJ03455J
Hassan AU, Sumrra SH, Imran M, Chohan ZH (2022) New 3d multifunctional metal chelates of sulfonamide: spectral, vibrational, molecular modeling, DFT, medicinal and in silico studies. J Mol Struct 1254:132305. https://doi.org/10.1016/j.molstruc.2021.132305
Quiroz-Garcia B, Figueroa R, Cogordan JA, Delgado G (2005) Photocyclodimers from Z-ligustilide. Experimental results and FMO analysis. Tetrahedron Lett 46:3003–3006
Friesen BA, Wiggins B, McHale JL et al (2010) Differing HOMO and LUMO mediated conduction in a porphyrin nanorod. J Am Chem Soc 132:8554–8556
Influence of energy gap between charge-transfer and locally excited states on organic long persistence luminescence | Nature Communications. https://www.nature.com/articles/s41467-019-14035-y. Accessed 19 Mar 2023
Hassan AU, Sumrra SH, Zafar MN et al (2022) New organosulfur metallic compounds as potent drugs: synthesis, molecular modeling, spectral, antimicrobial, drug likeness and DFT analysis. Mol Divers 26:51–72. https://doi.org/10.1007/s11030-020-10157-4
Hassan AU, Mohyuddin A, Güleryüz C et al (2022) Novel pull–push organic switches with D–π–A structural designs: computational design of star shape organic materials. Struct Chem 34(2):399–412. https://doi.org/10.1007/s11224-022-01983-3
Hassan AU, Sumrra SH, Zubair M et al (2022) Structurally modulated D-π-D-A(Semiconductor) anchoring dyes to enhance the tunable NLO response: a DFT/TDDFT quest for new photovoltaic materials. Struct Chem 34(3):1043–1060. https://doi.org/10.1007/s11224-022-02070-3
Yu J, Cui Y, Wu C et al (2012) Second-order nonlinear optical activity induced by ordered dipolar chromophores confined in the pores of an anionic metal–organic framework. Angew Chem 124:10694–10697
Durand RJ, Achelle S, Gauthier S et al (2018) Incorporation of a ferrocene unit in the $π$-conjugated structure of donor-linker-acceptor (D-$π$-A) chromophores for nonlinear optics (NLO). Dyes Pigments 155:68–74
Yahya M, Nural Y, Seferoğlu Z (2022) Recent advances in the nonlinear optical (NLO) properties of phthalocyanines: a review. Dyes Pig 198:109. https://doi.org/10.1016/j.dyepig.2021.109960
Hlel A, Mabrouk A, Chemek M et al (2015) A DFT study of charge-transfer and opto-electronic properties of some new materials involving carbazole units. Comput Condens Matter 3:30–40
Norman P, Bishop DM, Jensen HJA, Oddershede J (2005) Nonlinear response theory with relaxation: the first-order hyperpolarizability. J Chem Phys 123:194103
Sahki FA, Bouraiou A, Taboukhat S et al (2021) Design and synthesis of highly conjugated electronic phenanthrolines derivatives for remarkable NLO properties and DFT analysis. Optik 241:166. https://doi.org/10.1016/j.ijleo.2021.166949
Hassan AU, Mohyuddin A, Nadeem S et al (2022) Structural and electronic (absorption and fluorescence) properties of a stable triplet diphenylcarbene: a DFT study. J Fluoresc 32(5):1629–1638. https://doi.org/10.1007/s10895-022-02969-4
Chen C-Y, Wang M, Li J-Y et al (2009) Highly efficient light-harvesting ruthenium sensitizer for thin-film dye-sensitized solar cells. ACS Nano 3:3103–3109
Hassan AU, Sumrra SH (2022) Exploration of pull–push effect for novel photovoltaic dyes with A–π–D design: A DFT/TD-DFT investigation. J Fluoresc 32(6):1999–2014. https://doi.org/10.1007/s10895-022-03003-3
Singh Y, Patel RN, Patel SK et al (2019) Experimental and quantum computational study of two new bridged copper(II) coordination complexes as possible models for antioxidant superoxide dismutase: molecular structures, X-band electron paramagnetic spectra and cryogenic magnetic properties. Polyhedron 171:155–171. https://doi.org/10.1016/j.poly.2019.07.015
Hassan AU, Sumrra SH, Nazar MF, Güleryüz C (2022) A DFT study on new photovoltaic dyes to investigate their NLO tuning at near infrared region (NIR) as pull–push effect by end capped acceptors. J Fluoresc. https://doi.org/10.1007/s10895-022-03075-1
Galkowski K, Mitioglu A, Miyata A et al (2016) Determination of the exciton binding energy and effective masses for methylammonium and formamidinium lead tri-halide perovskite semiconductors. Energy Environ Sci 9:962–970. https://doi.org/10.1039/C5EE03435C
Hassan AU, Sumrra SH, Zafar W et al (2022) Enriching the compositional tailoring of NLO responsive dyes with diversity oriented electron acceptors as visible light harvesters: a DFT/TD-DFT approach. Mol Phys 121(1):e2148585. https://doi.org/10.1080/00268976.2022.2148585
Mary SJJ, Siddique MUM, Pradhan S et al (2020) Quantum chemical insight into molecular structure, NBO analysis of the hydrogen-bonded interactions, spectroscopic (FT--IR, FT--Raman), drug likeness and molecular docking of the novel anti COVID-19 molecule 2-[(4,6-diaminopyrimidin-2-yl)sulfanyl]-N-(4-fluorophenyl)acetamide - dimer. Spectrochim Acta A Mol Biomol Spectrosc 244:118825. https://doi.org/10.1016/j.saa.2020.118825
Sumrra SH, Arshad Z, Zafar W et al (2022) Metal incorporated aminothiazole-derived compounds: synthesis, density function theory analysis, in vitro antibacterial and antioxidant evaluation. R Soc Open Sci 8:210910. https://doi.org/10.1098/rsos.210910
Hassan AU, Sumrra SH (2022) Exploring the bioactive sites of new sulfonamide metal chelates for multi-drug resistance: an experimental versus theoretical design. J Inorg Organomet Polym Mater 32:513–535. https://doi.org/10.1007/s10904-021-02135-6
Hassan AU, Sumrra SH, Mustafa G, Noreen S, Ali A, Sara S, Imran M (2023) Enhancing NLO performance by utilizing tyrian purple dye as donor moiety in organic DSSCs with end capped acceptors: A theoretical study. J Mol Graph Model 124:108538. https://doi.org/10.1016/j.jmgm.2023.108538
Acknowledgements
The authors are grateful to the University of Gujrat, Gujrat, Pakistan, for the access to all research facilities.
Author information
Authors and Affiliations
Contributions
Conceptualization: Sajjad H. Sumrra; methodology: Abrar U. Hassan; formal analysis: Ayesha Mohyuddin; investigation: Muddassar Zafar; writing—original draft preparation: Sadaf Noreen; writing—review and editing: Cihat Güleryüz; resources: Sajjad H. Sumrra.
Corresponding authors
Ethics declarations
Ethics approval
This work did not involve any human subjects.
Consent to participate
This article does not have any studies with human participants or animals, clinical trial registration, or plant reproducibility performed by any author.
Consent for publication
All authors have approved the paper and agree with its publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hassan, A.U., Sumrra, S.H., Zafar, M. et al. DFT-guided structural modeling of end-group acceptors at Y123 core for sensitizers as high-performance organic solar dyes and NLO responses. J Mol Model 29, 262 (2023). https://doi.org/10.1007/s00894-023-05668-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05668-4