Abstract
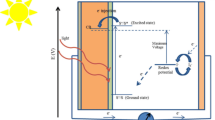
New organic dyes (H1-H6) for their promising applications as dye sensitized solar cell (DSSCs) were theoretically designed from 6,6′-di(thiophen-2-yl)-4,4′-bipyrimidine (TB) moiety in acceptor (A), π-spacer, and donor (D) type architecture to create A-π-D-π-D-π-A framework. After their benchmarking study against various exchange correlation (XC) and long-range corrected (LC) functional, the CAM-B3LYP produced accurate results so as selected for further density functional theory (DFT) and time dependent DFT (TD-DFT) studies. The frontier molecular orbitals (FMOs) were analyzed for their electron transfer properties, and their energies of the highest occupied molecular orbital (EHOMO) and the lowest unoccupied molecular orbital (ELUMO) were used to assess various electronic properties. Their energy differences (EH-L) ranged between 1.69 and 2.03 eV indicating their good responsiveness to electron injection (Ginj) being the values greater than 0.2 eV. The maximum absorbance of the dyes was reported around the 388–406 nm while their starting material (TB) had this value to be 371 nm. This redshift was significant with an intensification of electron-withdrawing groups on the dye structures. The dyes were evaluated for their device related parameters like their light harvesting efficiency, open circuit voltage, exciton binding energies, and exciton life times which were found in their promising values. The dyes were analyzed for their linear (α), first (βtotal), and second (γtotal) order nonlinear responses and were found to have significantly higher responses, not only against their starting materials but also those reported in literature for standard molecules (urea and p-nitric acid). Among the dyes under study, dye H4 exhibits the highest molecular polarizability (α) and hyperpolarizability (β and γ) values thanks to two CN substituted units on each ends. These dyes are most likely to have possible optical and photonic uses. Also, according to the results of the current theoretical research, all of these dyes may function as effective photosensitizer for their DSSC application.














Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information file.
Code availability
Gaussian 09 W and Gauss view 5.1 are used for simulation, and origin software is used to draw the plots.
References
Hassan AU, Mohyuddin A, Güleryüz C et al (2022) Novel pull–push organic switches with D–π–a structural designs: computational design of star shape organic materials. Struct Chem. https://doi.org/10.1007/s11224-022-01983-3
Richhariya G, Kumar A, Tekasakul P, Gupta B (2017) Natural dyes for dye sensitized solar cell: a review. Renew Sustain Energy Rev 69:705–718. https://doi.org/10.1016/j.rser.2016.11.198
O’Regan B, Grätzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353:737–740. https://doi.org/10.1038/353737a0
Meenakshi R (2017) Spectral investigations, DFT based global reactivity descriptors, inhibition efficiency and analysis of 5-chloro-2-nitroanisole as $π$-spacer with donor-acceptor variations effect for DSSCs performance. J Mol Struct 1127:694–707
Wang WV, Zhang Y, Li XY et al (2021) High performance nonvolatile organic field-effect transistor memory devices based on pyrene diimide derivative. InfoMat 3:814–822. https://doi.org/10.1002/inf2.12186
Meena M, Sundararajan RS, Manikandan E, Shalini M (2022) Diffractional, optical, electrical, NLO and surface studies of l-asparagine monohydrate lithium sulphate (LAMLS) single crystals. J Mater Sci Mater Electron. https://doi.org/10.1007/s10854-022-08734-4
Manfredi N, Cecconi B, Abbotto A (2014) Multi-branched multi-anchoring metal-free dyes for dye-sensitized solar cells. European J Org Chem 2014:7069–7086. https://doi.org/10.1002/ejoc.201402422
Sudarsan V (2012) Optical materials: fundamentals and applications. Funct Mater 285–322. https://doi.org/10.1016/B978-0-12-385142-0.00008-8
Nosheen E, Shah SM, Hussain H, Murtaza G (2016) Photo-sensitization of ZnS nanoparticles with renowned ruthenium dyes N3, N719 and Z907 for application in solid state dye sensitized solar cells: a comparative study. J Photochem Photobiol B Biol 162:583–591. https://doi.org/10.1016/j.jphotobiol.2016.07.033
Tachibana Y, Hara K, Sayama K, Arakawa H (2002) Quantitative analysis of light-harvesting efficiency and electron-transfer yield in ruthenium-dye-sensitized nanocrystalline TiO2 solar cells. Chem Mater 14:2527–2535
Duerto I, Sarasa S, Barrios D et al (2022) Enhancing the temporal stability of DSSCs with novel vinylpyrimidine anchoring and accepting group. Dye Pigment 203:110310. https://doi.org/10.1016/j.dyepig.2022.110310
Hassan AU, Sumrra SH (2022) Exploration of pull–push effect for novel photovoltaic dyes with a–π–D design: a DFT/TD-DFT investigation. J Fluoresc. https://doi.org/10.1007/s10895-022-03003-3
Noreen S, Sumrra SH (2022) Correlating the charge transfer efficiency of metallic sulfa-isatins to design efficient NLO materials with better drug designs. Biometals. https://doi.org/10.1007/s10534-022-00385-6
Sumrra SH, Arshad Z, Zafar W et al (2022) Metal incorporated aminothiazole-derived compounds: synthesis, density function theory analysis, in vitro antibacterial and antioxidant evaluation. R Soc Open Sci 8:210910. https://doi.org/10.1098/rsos.210910
Reynal A, Palomares E (2011) Ruthenium polypyridyl sensitisers in dye solar cells based on mesoporous TiO2. Eur J Inorg Chem 2011:4509–4526. https://doi.org/10.1002/ejic.201100516
Abdel-Kader NS, Abdel-Latif SA, El-Ansary AL, Sayed AG (2021) Spectroscopic studies, density functional theory calculations, non-linear optical properties, biological activity of 1-hydroxy-4-((4-(N-(pyrimidin-2-yl)sulfamoyl)phenyl)diazenyl)-2-naphthoic acid and its chelates with Nickel (II), Copper (II), Zinc (II) a. J Mol Struct 1223:129203. https://doi.org/10.1016/j.molstruc.2020.129203
Tao J, Vignale G, Tokatly IV (2007) Time-dependent density functional theory: derivation of gradient-corrected dynamical exchange-correlational potentials. Phys Rev B 76:195126. https://doi.org/10.1103/PhysRevB.76.195126
Jacquemin D, Perpète EA, Scalmani G et al (2007) Assessment of the efficiency of long-range corrected functionals for some properties of large compounds. J Chem Phys 126:144105. https://doi.org/10.1063/1.2715573
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393:51–57
Ma LJ, Jia J, Wu HS, Ren Y (2013) Ti–η2-(C2H2) and HCC–TiH as high capacity hydrogen storage media. Int J Hydrogen Energy 38:16185–16192. https://doi.org/10.1016/j.ijhydene.2013.09.151
Rayne S, Forest K (2016) A comparative examination of density functional performance against the ISOL24/11 isomerization energy benchmark. Comput Theor Chem 1090:147–152
Hassan AU, Sumrra SH, Zafar W et al (2022) Enriching the compositional tailoring of NLO responsive dyes with diversity oriented electron acceptors as visible light harvesters: a DFT/TD-DFT approach. Mol Phys e2148585. https://doi.org/10.1080/00268976.2022.2148585
Huang Y, Tong L (2022) 6,6′-Di-(2″-thiophenol)-2,2′-bipyridine. Molbank 2022
Manzoni V, Gester R, da Cunha AR et al (2021) Solvent effects on Stokes shifts, and NLO response of thieno[3,4-b]pyrazine: a comprehensive QM/MM investigation. J Mol Liq 335:115996. https://doi.org/10.1016/j.molliq.2021.115996
Almogati RN, Aziz SG, Hilal R (2017) Effect of substitution on the optoelectronic properties of dyes for DSSC. A DFT approach. J Theor Comput Chem 16:1750018. https://doi.org/10.1142/S0219633617500183
Noreen S, Sumrra SH (2021) Aminothiazole-linked metal chelates: synthesis, density functional theory, and antimicrobial studies with antioxidant correlations. ACS Omega 6:33085–33099
Galkowski K, Mitioglu A, Miyata A et al (2016) Determination of the exciton binding energy and effective masses for methylammonium and formamidinium lead tri-halide perovskite semiconductors. Energy Environ Sci 9:962–970. https://doi.org/10.1039/C5EE03435C
Gangadhar PS, Jagadeesh A, Rajesh MN et al (2022) Role of π-spacer in regulating the photovoltaic performance of copper electrolyte dye-sensitized solar cells using triphenylimidazole dyes. Mater Adv 3:1231–1239. https://doi.org/10.1039/D1MA00852H
Hassan AU, Sumrra SH, Mustafa G et al (2022) Efficient and tunable enhancement of NLO performance for indaceno-based donor moiety in A-π-D-π-D-π-A type first DSSC design by end-capped acceptors. J Mol Model 29:4. https://doi.org/10.1007/s00894-022-05402-6
Bhattacharya S, Biswas C, Raavi SSK et al (2019) Synthesis, optical, electrochemical, DFT studies, NLO properties, and ultrafast excited state dynamics of carbazole-induced phthalocyanine derivatives. J Phys Chem C 123:11118–11133
Krishna JVS, Krishna NV, Chowdhury TH et al (2018) Kinetics of dye regeneration in liquid electrolyte unveils efficiency of 10.5% in dye-sensitized solar cells. J Mater Chem C 6:11444–11456. https://doi.org/10.1039/C8TC04370A
De Angelis F, Fantacci S, Selloni A et al (2010) First-principles modeling of the adsorption geometry and electronic structure of Ru(II) dyes on extended TiO2 substrates for dye-sensitized solar cell applications. J Phys Chem C 114:6054–6061. https://doi.org/10.1021/jp911663k
Zhang J, Li HB, Sun SL et al (2012) Density functional theory characterization and design of high-performance diarylamine-fluorene dyes with different π spacers for dye-sensitized solar cells. J Mater Chem 22:568–576. https://doi.org/10.1039/C1JM13028E
Tunç G, Güzel E, Şişman İ et al (2019) Effect of new asymmetrical Zn(ii) phthalocyanines on the photovoltaic performance of a dye-sensitized solar cell. New J Chem 43:14390–14401. https://doi.org/10.1039/C9NJ02585E
Hassan AU, Guleryuz C (2021) Theoretical evaluation of the permeability of the discharge item (LiOOH) IN Li-O-2 batteries. Lat Am Appl Res 51:153–157
Hassan AU, Sumrra SH (2022) Exploring the bioactive sites of new sulfonamide metal chelates for multi-drug resistance: an experimental versus theoretical design. J Inorg Organomet Polym Mater 32:513–535. https://doi.org/10.1007/s10904-021-02135-6
Hassan AU, Mohyuddin A, Nadeem S et al (2022) Structural and electronic (absorption and fluorescence) properties of a stable triplet diphenylcarbene: a DFT study. J Fluoresc. https://doi.org/10.1007/s10895-022-02969-4
Mintmire JW, White CT (1998) Universal density of states for carbon nanotubes. Phys Rev Lett 81:2506
Hassan AU, Sumrra SH, Zafar MN et al (2022) New organosulfur metallic compounds as potent drugs: synthesis, molecular modeling, spectral, antimicrobial, drug likeness and DFT analysis. Mol Divers 26:51–72. https://doi.org/10.1007/s11030-020-10157-4
Jackson N, Czaplewski L, Piddock LJV (2018) Discovery and development of new antibacterial drugs: learning from experience? J Antimicrob Chemother 73:1452–1459. https://doi.org/10.1093/jac/dky019
Greisch JF, Harding ME, Kordel M et al (2013) Intrinsic fluorescence properties of rhodamine cations in gas-phase: triplet lifetimes and dispersed fluorescence spectra. Phys Chem Chem Phys 15:8162–8170. https://doi.org/10.1039/C3CP44362K
Sumrra SH, Hassan AU, Zafar MN et al (2022) Metal incorporated sulfonamides as promising multidrug targets: combined enzyme inhibitory, antimicrobial, antioxidant and theoretical exploration. J Mol Struct 1250:131710. https://doi.org/10.1016/j.molstruc.2021.131710
Vamsikrishna N, Kumar MP, Ramesh G et al (2017) REGULAR ARTICLE DNA interactions and biocidal activity of metal complexes of benzothiazole Schiff bases : synthesis, characterization. J Chem Sci. https://doi.org/10.1007/s12039-017-1273-7
Zhang F, Wu D, Xu Y, Feng X (2011) Thiophene-based conjugated oligomers for organic solar cells. J Mater Chem 21:17590–17600. https://doi.org/10.1039/C1JM12801A
Akin S, Açikgöz S, Gülen M et al (2016) Investigation of the photoinduced electron injection processes for natural dye-sensitized solar cells: the impact of anchoring groups. RSC Adv 6:85125–85134
Islam MN, Haque MA (1982) The lateral photovoltaic effect in CdS-Cu2S heterojunction solar cell. Appl Phys A 28:145–149. https://doi.org/10.1007/BF00617146
Guechtouli N, Zaater S, Kichou N et al (2019) Theoretical investigation of dicarboxamide mono copper (II) and novel transition metal complexes: structural, chemical reactivity, vibrational and in-silico biological analysis. J Mol Struct 1188:23–30. https://doi.org/10.1016/j.molstruc.2019.03.068
Aydın G, Koçak O, Güleryüz C, Yavuz I (2020) Structural order and charge transfer in highly strained carbon nanobelts. New J Chem 44:15769–15775. https://doi.org/10.1039/D0NJ03455J
Hassan AU, Sumrra SH, Nazar MF, Güleryüz C (2022) A DFT study on new photovoltaic dyes to investigate their NLO tuning at near infrared region (NIR) as pull–push effect by end capped acceptors. J Fluoresc. https://doi.org/10.1007/s10895-022-03075-1
Zou G, Ok KM (2020) Novel ultraviolet (UV) nonlinear optical (NLO) materials discovered by chemical substitution-oriented design. Chem Sci 11:5404–5409
Garmire E (2013) Nonlinear optics in daily life. Opt Express 21:30532–30544. https://doi.org/10.1364/OE.21.030532
Hassan AU, Sumrra SH, Nkungli NK, Güleryüz C (2022) Theoretical probing of 3d nano metallic clusters as next generation non-linear optical materials. Results Chem 100627. https://doi.org/10.1016/j.rechem.2022.100627
El-Shafiy HF, Shebl M (2019) Binuclear oxovanadium(IV), cerium(III) and dioxouranium(VI) nano complexes of a bis(bidentate) ligand: synthesis, spectroscopic, thermal, DFT calculations and biological studies. J Mol Struct 1194:187–203. https://doi.org/10.1016/j.molstruc.2019.05.063
Hassan AU, Sumrra SH, Imran M, Chohan ZH (2022) New 3d multifunctional metal chelates of sulfonamide: spectral, vibrational, molecular modeling, DFT, medicinal and in silico studies. J Mol Struct 132305. https://doi.org/10.1016/j.molstruc.2021.132305
Acknowledgements
The authors are grateful to the University of Gujrat, Gujrat, Pakistan, for accessing them all the available research facilities. MI extends his appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Large Group Research Project under grant number 34/43 and also acknowledges the Research Center for Advance Materials (RCAMS) at King Khalid University, Saudi Arabia for their valuable technical support.
Author information
Authors and Affiliations
Contributions
Conceptualization: Sajjad H. Sumrra; methodology: Abrar U. Hassan; formal analysis and investigation: Ghulam Mustafa and Muhammad Zubair; writing-original draft preparation: Sadaf Noreen; writing-review and editing: Ayesha Mohyuddin; resources: Muhammad Imran.
Corresponding authors
Ethics declarations
Ethical approval
This work did not involve any human subjects.
Consent for publication
All authors have approved the paper and agree with its publication.
Consent to participate
This article does not have any studies with human participants or animals, clinical trial registration, or plant reproducibility performed by any author.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hassan, A.U., Sumrra, S.H., Mustafa, G. et al. Creating intense and refined NLO responses by utilizing dual donor structural designs in A-π-D-π-D-π-A type organic switches: computed device parameters. Struct Chem 34, 2021–2038 (2023). https://doi.org/10.1007/s11224-023-02138-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-023-02138-8