Abstract
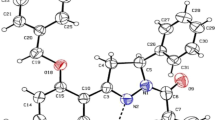
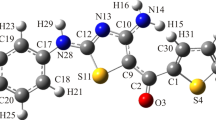
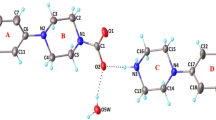
Dihydropyridines are the most extensively used drugs in the treatment of hypertension. Nifedipine is the prototype of calcium channel blocker. The dihydropyridine derivative compounds of diethyl 4-(4-bromophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate (DHPB), diethyl 4-(furan-2yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate (DHPF), and diethyl-4-phenyl-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate (DHPP) were synthesized using the Hantzsch reaction. The DFT/B3LYP exchange–correlation function was employed to perform quantum chemical calculations such as molecular geometry optimization, vibrational analysis, frontier molecular orbital (FMO), molecular electrostatic potential (MEP), natural bond order (NBO), global reactive descriptors, and Fukui functions to determine the structural characteristics related to biological activity of the compounds. The molecular docking and molecular dynamics were employed to study the binding interaction and stability of protein–ligand complex in the docked site.









Similar content being viewed by others
Data availability
The data used in this study are available from the corresponding author upon interest.
References
Mohamed MF, Ibrahim NS, Elwahy AHM, Abdelhamid IA (2018) Molecular studies on novel antitumor bis 1,4-dihydropyridine derivatives against lung carcinoma and their limited side effects on normal melanocytes. Anti-Cancer Agents Med Chem (Formerly Curr Med Chem Agents) 18:2156–2168
Briukhanov VM, Zverev I, Elkin VI (1994) The effect of calcium antagonists on the development of inflammatory edema in rats. Eksp Klin Farmakol 57:47–49
Loev B, Goodman MM, Snader KM et al (1974) Hantzsch-type dihydropyridine hypotensive agents. J Med Chem 17:956–965
Bossert F, Meyer H, Wehinger E (1981) 4-Aryldihydropyridines, a new class of highly active calcium antagonists. Angew Chemie Int Ed English 20:762–769
Breitenbucher JG, Figliozzi G (2000) Solid-phase synthesis of 4-aryl-1, 4-dihydropyridines via the Hantzsch three component condensation. Tetrahedron Lett 41:4311–4315
Triggle DJ (2007) Calcium channel antagonists: clinical uses—past, present and future. Biochem Pharmacol 74:1–9
Wächter GA, Davis MC, Martin AR, Franzblau SG (1998) Antimycobacterial activity of substituted isosteres of pyridine-and pyrazinecarboxylic acids. J Med Chem 41:2436–2438
Sunkel CE, Fau de Casa-Juana M, Santos L et al (1990) 4-Alkyl-1, 4-dihydropyridine derivatives as specific PAF-acether antagonists. J Med Chem 33:3205–3210
Tusell JM, Barro S, Serratosa J (1993) Anticonvulsant activity of δ-HCH, calcium channel blockers and calmodulin antagonists in seizures induced by lindane and other convulsant drugs. Brain Res 622:99–104
Budriesi R, Ioan P, Locatelli A et al (2008) Imidazo[2,1-b]thiazole system: a scaffold endowing dihydropyridines with selective cardiodepressant activity. J Med Chem 51:1592–1600
Xu L, Li D, Tao L et al (2016) Binding mechanisms of 1,4-dihydropyridine derivatives to L-type calcium channel Ca v 1.2: a molecular modeling study. Mol Biosyst 12:379–390
Birnbaumer L, Campbell KP, Catterall WA et al (1994) The naming of voltage-gated calcium channels. Neuron 13:505–506
Hantzsch A (1882) Ueber die synthese pyridinartiger verbindungen aus acetessigäther und aldehydammoniak. Justus Liebigs Ann Chem 215:1–82
Gaussian09 RA (2009) 1, mj frisch, gw trucks, hb schlegel, ge scuseria, ma robb, jr cheeseman, g. Scalmani, v. Barone, b. Mennucci, ga petersson et al., gaussian. Inc, Wallingford CT 121:150–166
Dennington RD, Keith TA, Millam JM (2008) GaussView 5.0. Gaussian, Inc, Wallingford
Jamroz MH (2004) Vibrational energy distribution analysis VEDA 4
Weinhold F, Landis CR (2001) Natural bond orbitals and extensions of localized bonding concepts. Chem Educ Res Pract 2:91–104
Morris GM, Huey R, Lindstrom W et al (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791
Schrodinger LLC (2010) The PyMOL molecular graphics system. Version 1:0
Schrodinger LLC (2019) Schrodinger Release 2019–3: Desmond Molecular Dynamics System. DE Shaw Research. Maestro-Desmond Interoperability Tools, Schrodinger, LLC, New York
Boulcina R, Bouacida S, Roisnel T, Debache A (2007) Diethyl 4-(4-bromophenyl)-2, 6-dimethyl-1, 4-dihydropyridine-3, 5-dicarboxylate. Acta Crystallogr Sect E Struct Reports Online 63:o3635–o3636
Sagar MB, Ravikumar K, Sadanandam YS (1999) Crystal and molecular structure of 2, 6-dimethyl-3, 5-di [N-methyl]-carbamoyl-4-[3, 4-methoxy] phenyl-1, 4-dihydropyridine hemihydrate and 2, 6-dimethyl-3, 5-di [N-methyl] carbamoyl-4-[furan]-1, 4-dihydropyridine
Bai M-S, Chen Y-Y, Niu D-L, Peng L (2009) Diethyl 2, 6-dimethyl-4-phenyl-1, 4-dihydropyridine-3, 5-dicarboxylate. Acta Crystallogr Sect E Struct Reports Online 65:o799–o799
Kavitha T, Velraj G (2016) Structural, spectroscopic (FT-IR, FT-Raman, NMR) and computational analysis (DOS, NBO, Fukui) of 3, 5-dimethylisoxazole and 4-(chloromethyl)-3, 5-dimethylisoxazole: a DFT study. J Theor Comput Chem 15:1650039
Chung TW, Tureček F (2008) Electronic properties of charge-tagged peptides upon electron capture. Eur J Mass Spectrom 14:367–378
Scott AP, Radom L (1996) Harmonic vibrational frequencies: an evaluation of Hartree−Fock, Møller−Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J Phys Chem 100:16502–16513
Kalsi PS (2007) Spectroscopy of organic compounds. New Age International
Govindasamy P, Gunasekaran S (2015) Quantum mechanical calculations and spectroscopic (FT-IR, FT-Raman and UV) investigations, molecular orbital, NLO, NBO, NLMO and MESP analysis of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl] benzene-1-sulfonamide. J Mol Struct 1081:96–109
Johnson RD (2006) NIST computational chemistry comparison and benchmark database. http://srdata.nist.gov/cccbdb. Accessed 15 Mar 2021
Turan N, Buldurun K, Alan Y et al (2019) Synthesis, characterization, antioxidant, antimicrobial and DNA binding properties of ruthenium(II), cobalt(II) and nickel(II) complexes of Schiff base containing o-vanillin. Res Chem Intermed 45:3525–3540. https://doi.org/10.1007/s11164-019-03806-3
Chaitanya K (2012) Molecular structure, vibrational spectroscopic (FT-IR, FT-Raman), UV–vis spectra, first order hyperpolarizability, NBO analysis, HOMO and LUMO analysis, thermodynamic properties of benzophenone 2, 4-dicarboxylic acid by ab initio HF and density functional. Spectrochim Acta Part A Mol Biomol Spectrosc 86:159–173
Uthayakumar M, Jeyakumari AP, Dhandapani A, et al (2019) Synthesis, experimental and computational spectroscopic investigations of third-order nonlinear optical material (E)-N′-(benzo[d][1,3]dioxol-5-ylmethylene)benzohydrazide. J Phys D Appl Phys 52: https://doi.org/10.1088/1361-6463/ab284b
Koopmans T (1934) Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den einzelnen Elektronen eines Atoms. Physica 1:104–113
Sridevi C, Shanthi G, Velraj G (2012) Structural, vibrational, electronic, NMR and reactivity analyses of 2-amino-4H-chromene-3-carbonitrile (ACC) by ab initio HF and DFT calculations. Spectrochim Acta Part A Mol Biomol Spectrosc 89:46–54
Lakshmi A, Balachandran V, Janaki A (2011) Comparative vibrational spectroscopic studies, HOMO–LUMO and NBO analysis of 5,7-dibromo-8-hydroxyquinoline and 5,7-dichloro-8-hydroxyquinoline based on Density Functional Theory. J Mol Struct 1004:51–66
Thul P, Gupta VP, Ram VJ, Tandon P (2010) Structural and spectroscopic studies on 2-pyranones. Spectrochim Acta Part A Mol Biomol Spectrosc 75:251–260
Scrocco E, Tomasi J (1978) Electronic molecular structure, reactivity and intermolecular forces: an euristic interpretation by means of electrostatic molecular potentials. In: Advances in quantum chemistry. Elsevier, 115–193
Luque FJ, López JM, Orozco M (2000) Perspective on “Electrostatic interactions of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects.” Theor Chem Acc 103:343–345
James C, Ravikumar C, Sundius T et al (2008) FT-Raman and FTIR spectra, normal coordinate analysis and ab initio computations of (2-methylphenoxy) acetic acid dimer. Vib Spectrosc 47:10–20
Kavitha T, Velraj G (2017) Density functional theory analysis and molecular docking evaluation of 1-(2, 5-dichloro-4-sulfophenyl)-3-methyl-5-pyrazolone as COX2 inhibitor against inflammatory diseases. J Mol Struct. https://doi.org/10.1016/j.molstruc.2017.03.061
Fleming I (1977) Frontier orbitals and organic chemical reactions. Wiley
Parr RG (1980) Density functional theory of atoms and molecules. In: Horizons of quantum chemistry. Springer, pp 5–15
Lehr GF, Lawler RG (1984) Quantitative CIDNP evidence for the SH2 reaction of alkyl radicals with Grignard reagents. Implication to the iron catalyzed Kharasch reaction. J Am Chem Soc 106:4048–4049
Jorgensen WL (2004) The many roles of computation in drug discovery. Science (80-) 303:1813–1818
Molinspiration C (2011) Calculation of molecular properties and bioactivity score. http://www.molinspiration.com/cgi-bin/properties. Accessed 18 Mar 2021
Daina A, Michielin O, Zoete V (2019) SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res 47:W357–W364. https://doi.org/10.1093/nar/gkz382
Tang L, El-Din TMG, Swanson TM et al (2016) Structural basis for inhibition of a voltage-gated Ca 2+ channel by Ca 2+ antagonist drugs. Nature 537:117–121
Sebhaoui J, El Bakri Y, Lai CH, et al (2021) Unexpected synthesis of novel 2-pyrone derivatives: crystal structures, Hirshfeld surface analysis and computational studies. J Biomol Struct Dyn 39:4859–4877. https://doi.org/10.1080/07391102.2020.1780943
El Bakri Y, Anouar EH, Subramani K et al (2020) Synthesis, spectroscopic characterizations, DFT, molecular docking and molecular dynamics simulations of a novel 2-methyl-3H-benzimidazolo[1,2-b][1,2,4]triazepin-4(5H)-one. J Mol Struct 1202:127317. https://doi.org/10.1016/j.molstruc.2019.127317
Acknowledgements
The authors gratefully acknowledge the Anna University, Chennai, and IIT Madras for providing facilities FT-IR, FT-Raman, and UV-Vis spectrometer to record the spectra of the samples.
Author information
Authors and Affiliations
Contributions
R. Karthick and S. Karthikeyan have performed simulation studies, and M. P. Pachamuthu has carried out synthesis of compounds. All these work are done under the guidance of Dr. G. Velraj.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karthick, R., Velraj, G., Pachamuthu, M.P. et al. Synthesis, spectroscopic, DFT, and molecular docking studies on 1,4-dihydropyridine derivative compounds: a combined experimental and theoretical study. J Mol Model 28, 5 (2022). https://doi.org/10.1007/s00894-021-04939-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04939-2