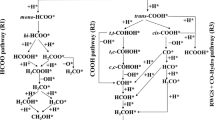
Abstract
Cu-based catalysts have been widely used for water-gas shift reaction (WGS, CO + H2O → CO2 + H2), and α-MoC support also shows the good performance for the reaction. Therefore, WGS reaction is systematically studied over Cu/α-MoC by using density functional theory (DFT). DFT result shows the strong metal-support interaction between Cu and α-MoC(111) support. As a result, an extensive tensile strain is introduced in the Cu lattice due to α-MoC support, and Cu 3d band center shifts to Fermi level. However, the strong metal-support interaction does not lead to significant polarization of the Cu/α-MoC surface due to the less charge transfer from Mo to Cu. For the WGS reaction, small Cu particles on α-MoC(111) are likely to facilitate the reaction. At the interface of Cu-α-MoC(111), oxygen stabilizes the dissociated *H, which is benefit of H2O scission. Then, the activity increases compared with Cu(111) surface. In general, small Cu particles on α-MoC support also have good activity for WGS reaction compared with Au deposition on α-MoC.

Graphical abstract




Similar content being viewed by others
References
Zhu M, Wachs IE (2016) Iron-based catalysts for the high-temperature water–gas shift (HT-WGS) reaction. ACS Catal 6:722–732
Zhang Y, Cai J, Liu Y, Wang X, Au C-T, Jiang L (2019) Preparation of sintering-resistant Pt nanocatalysts by dopamine mediation for water-gas shift reaction. Appl Surf Sci 496:143669
Sun SD, Zhang X, Cui J, Yang Q, Liang SH (2019) Tuning interfacial Cu–O atomic structures for enhanced catalytic applications. Chem Asian J 14:2912–2924
Shim J-O, Na H-S, Ahn S-Y, Jeon K-W, Jang W-J, Jeon B-H, Roh H-S (2019) An important parameter for synthesis of Al2O3 supported Cu-Zn catalysts in low-temperature water-gas shift reaction under practical reaction condition. Int J Hydrog Energy 44:14853–14860
Kowalik P, Antoniak-Jurak K, Bicki R, Próchniak W, Wiercioch P, Michalska K (2019) The alcohol-modified CuZnAl hydroxycarbonate synthesis as a convenient preparation route of high activity Cu/ZnO/Al2O3 catalysts for WGS. Int J Hydrog Energy 44:913–922
Rodriguez JA, Grinter DC, Liu Z, Palomino RM, Senanayake SD (2017) Ceria-based model catalysts: fundamental studies on the importance of the metal–ceria interface in CO oxidation, the water–gas shift, CO2 hydrogenation, and methane and alcohol reforming. Chem Soc Rev 46:1824–1841
Jampaiah D, Damma D, Chalkidis A, Singh M, Sabri YM, Mayes ELH, Bansal V, Bhargava SK (2019) MOF-derived noble-metal-free Cu/CeO2 with high porosity for the efficient water–gas shift reaction at low temperatures. Catal Sci Technol 9:4226–4231
Zhou Y, Li Y, Shen WJ (2016) Shape engineering of oxide nanoparticles for heterogeneous catalysis. Chem Asian J 11:1470–1488
Wu H-C, Chen T-C, Wu J-H, Chen C-H, Lee J-F, Chen C-S (2016) The effect of an Fe promoter on Cu/SiO2 catalysts for improving their catalytic activity and stability in the water-gas shift reaction. Catal Sci Technol 6:6087–6096
Mo L, Saw E-T, Kathiraser Y, Ang ML, Kawi S (2018) Preparation of highly dispersed Cu/SiO2 doped with CeO2 and its application for high temperature water gas shift reaction. Int J Hydrog Energy 43:15891–15897
Rodriguez JA, Illas F (2012) Activation of noble metals on metal-carbide surfaces: novel catalysts for CO oxidation, desulfurization and hydrogenation reactions. Phys Chem Chem Phys 14:427–438
Hwu HH, Chen JG (2005) Surface chemistry of transition metal carbides. Chem Rev 105:185–212
Zhang Q, Pastor-Pérez L, Jin W, Gu S, Reina TR (2019) Understanding the promoter effect of Cu and Cs over highly effective β-Mo2C catalysts for the reverse water-gas shift reaction. Appl Catal B 244:889–898
Xu W, Ramirez PJ, Stacchiola D, Rodriguez JA (2014) Synthesis of α-MoC1-x and β-MoCy catalysts for CO2 hydrogenation by thermal carburization of Mo-oxide in hydrocarbon and hydrogen mixtures. Catal Lett 144:1418–1424
Yao S, Zhang X, Zhou W, Gao R, Xu W, Ye Y, Lin L, Wen X, Liu P, Chen B et al (2017) Atomic-layered Au clusters on α-MoC as catalysts for the low-temperature water-gas shift reaction. Science 357:389–393
Sabnis KD, Cui Y, Akatay MC, Shekhar M, Lee WS, Miller JT, Delgass WN, Ribeiro FH (2015) Water–gas shift catalysis over transition metals supported on molybdenum carbide. J Catal 331:162–171
Kresse G, Furthmüller (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. J Phys Rev B 54: 11169–11186
Blöchl PE (1994) Projector augmented-wave method. Phys Rev B 50:17953–17979
Kresse G, Hafner J (1993) Ab initio molecular dynamics for liquid metals. Phys Rev B 47:558–561
Ernzerhof M, Scuseria GE (1999) Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. J Chem Phys 110:5029–5036
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Posada-Pérez S, Viñes F, Rodríguez JA, Illas FJ (2015) Structure and electronic properties of Cu nanoclusters supported on Mo2C(001) and MoC(001) surfaces. Chem Phys 143:114704
Henkelman G, Uberuaga BP, Jónsson HJ (2000) A climbing image nudged elastic band method for finding saddle points and minimum energy paths. Chem Phys 113:9901–9904
Milman V, Winkler B, White JA, Pickard CJ, Payne MC, Akhmatskaya EV, Nobes RH (2000) Electronic structure, properties, and phase stability of inorganic crystals: a pseudopotential plane-wave study. Int J Quantum Chem 77:895–910
Fernández Guillermet A, Häglund J, Grimvall G (1992) Cohesive properties of 4d-transition-metal carbides and nitrides in the NaCl-type structure. Phys Rev B 45:11557–11567
Liu P, Rodriguez JA (2006) Water-gas-shift reaction on molybdenum carbide surfaces: essential role of the oxycarbide. J Phys Chem B 110:19418–19425
Liu P, Rodriguez JA (2007) Water-gas-shift reaction on metal nanoparticles and surfaces. J Chem Phys 126:164705
Nakamura J, Campbell JM, Campbell CT (1990) Kinetics and mechanism of the water-gas shift reaction catalysed by the clean and Cs-promoted Cu(110) surface: a comparison with Cu(111). J Chem Soc Faraday Trans 86:2725–2734
Wang YX, Wang GC (2019) A systematic theoretical study of water gas shift reaction on Cu(111) and Cu(110): potassium effect. ACS Catal 9:2261–2274
Wang GC, Nakamura J (2010) Structure sensitivity for forward and reverse water-gas shift reactions on copper surfaces: a DFT study. J Phys Chem Lett 1:3053–3057
Plata JJ, Graciani J, Evans J, Rodriguez JA, Sanz JF (2016) Cu deposited on CeOx-modified TiO2(110): synergistic effects at the metal–oxide Interface and the mechanism of the WGS reaction. ACS Catal 6:4608–4615
Zuo Z-J, Wang L, Han P-D, Huang W (2014) Insights into the reaction mechanisms of methanol decomposition, methanol oxidation and steam reforming of methanol on Cu(111): a density functional theory study. Int J Hydrog Energy 39:1664–1679
Liu Y-M, Liu J-T, Liu S-Z, Li J, Gao Z-H, Zuo Z-J, Huang WJ (2017) Reaction mechanisms of methanol synthesis from CO/CO2 hydrogenation on Cu2O(111): comparison with Cu(111). J CO2 Util 20:59–65
Grabow LC, Mavrikakis M (2011) Mechanism of methanol synthesis on cu through CO2 and CO hydrogenation. ACS Catal 1:365–384
Wang GC, Jiang L, Cai ZS, Pan YM, Zhao XZ, Huang W, Xie KC, Li YW, Sun YH, Zhong B (2003) Surface structure sensitivity of the water-gas shift reaction on Cu(hkl) surfaces: a theoretical study. J Phys Chem B 107:557–562
Tauster SJ (1987) Strong metal-support interactions. Acc Chem Res 20:389–394
Campbell CT (2012) Catalyst-support interactions: electronic perturbations. Nat Chem 4:597–598
Bruix A, Rodriguez JA, Ramírez PJ, Senanayake SD, Evans J, Park JB, Stacchiola D, Liu P, Hrbek J, Illas F (2012) A new type of strong metal–support interaction and the production of H2 through the transformation of water on Pt/CeO2(111) and Pt/CeOx/TiO2(110) catalysts. J Am Chem Soc 134:8968–8974
Mavrikakis M, Hammer B, Norskov JK (1998) Effect of strain on the reactivity of metal surfaces. Phys Rev Lett 81:2819–2822
Mamatkulov M, Filhol JS (2011) An ab initio study of electrochemical vs. electromechanical properties: the case of CO adsorbed on a Pt(111) surface. Phys Chem Chem Phys 13:7675–7684
Rodriguez JA, Liu P, Viñes JF, Illas F, Takahashi Y, Nakamura K (2008) Dissociation of SO2 on Au/TiC(001): effects of Au↔C interactions and charge polarization. Angew Chem Int Ed 47:6685–6689
Vidal AB, Feria L, Evans J, Takahashi Y, Liu P, Nakamura K, Illas F, Rodriguez JA (2012) CO2 activation and methanol synthesis on novel Au/TiC and Cu/TiC catalysts. J Phys Chem Lett 3:2275–2280
Funding
The authors would like to thank the National Natural Science Foundation of China (Nos. 21776197 and 21776195), Scientific and Technological Key Project of Shanxi Province (No. 20191102003), and Key R&D program of Shanxi Province (International Cooperation, No. 201903D421074) for their financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1209 kb)
Rights and permissions
About this article
Cite this article
Zou, XY., Mi, L., Zuo, ZJ. et al. DFT study the water-gas shift reaction over Cu/α-MoC surface. J Mol Model 26, 237 (2020). https://doi.org/10.1007/s00894-020-04502-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04502-5