Abstract
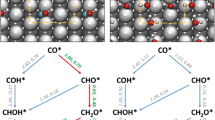
In this paper, the density functional theory calculation is used to investigate the reaction mechanisms of CO2 hydrogenation to CH3OH on the Cu@Pd core–shell surface. In particular, the possible adsorption sites, structural parameters, and adsorption energies of all intermediates are determined. All the reaction energies and the activation barriers of elementary steps involved in the possible hydrogenation mechanisms, including the HCOO pathway, the COOH pathway, and the RWGS + CO-Hydro pathway, are studied in detail. The calculated activation barrier of the rate-determining step is 1.84 eV for the HCOO pathway, 1.45 eV for the COOH pathway, and 1.17 eV for the RWGS + CO-Hydro pathway. Therefore, the most favorable hydrogenation mechanism on the Cu@Pd core–shell surface is following the reverse water–gas shift reaction followed by CO hydrogenation and will be completed via the route of CO2* → trans-COOH* → cis-COOH* → CO* → HCO* → HCOH* → H2COH* → CH3OH*. Compared with Cu-based catalysts such as Cu(111), Cu29 cluster, Cu19 cluster, and Cu(211), the Cu@Pd core–shell catalyst is demonstrated to have higher catalytic activity toward CO2 hydrogenation to CH3OH.







Similar content being viewed by others
References
Podjaski F, Kröger J, Lotsch BV (2018) Toward an aqueous solar battery: direct electrochemical storage of solar energy in carbon nitrides. Adv Mater 30:1705477
Zheng X, Cao X, Sun Z et al (2020) Indiscrete metal/metal-N-C synergic active sites for efficient and durable oxygen electrocatalysis toward advanced Zn-air batteries. Appl Catal B Environ 272:118967
Chen X, Chang J, Ke Q (2018) Probing the activity of pure and N-doped fullerenes towards oxygen reduction reaction by density functional theory. Carbon 126:53–57
Chen X, Sun F, Bai F, Xie Z (2019) DFT study of the two dimensional metal–organic frameworks X3(HITP)2 as the cathode electrocatalysts for fuel cell. Appl Surf Sci 471:256–262
Zhang L, Shan B, Zhao Y, Guo Z (2019) Review of micro seepage mechanisms in shale gas reservoirs. Int J Heat Mass Transfer 139:144–179
Zhang L, Chen Z, Zhao Y (2019) Well production performance analysis for shale gas reservoirs. Elsevier, Cambridge
Wang W-H, Himeda Y, Muckerman JT, Manbeck GF, Fujita E (2015) CO2 hydrogenation to formate and methanol as an alternative to photo- and electrochemical CO2 reduction. Chem Rev 115:12936–12973
Appel AM, Bercaw JE, Bocarsly AB et al (2013) Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation. Chem Rev 113:6621–6658
Aresta M, Dibenedetto A (2007) Utilisation of CO2 as a chemical feedstock: opportunities and challenges. Dalton Trans 28:2975–2992
Aresta M, Dibenedetto A, Angelini A (2013) Catalysis for the valorization of exhaust carbon: from CO2 to chemicals, materials, and fuels. Technological use of CO2. Chem Rev 114:1709–1742
Ansari MB, Park S-E (2012) Carbon dioxide utilization as a soft oxidant and promoter in catalysis. Energy Environ Sci 5:9419–9437
Wang W, Wang S, Ma X, Gong J (2011) Recent advances in catalytic hydrogenation of carbon dioxide. Chem Soc Rev 40:3703–3727
Song C (2006) Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal Today 115:2–32
Xu X, Moulijn JA (1996) Mitigation of CO2 by chemical conversion: plausible chemical reactions and promising products. Energy Fuels 10:305–325
Mikkelsen M, Jørgensen M, Krebs FC (2010) The teraton challenge. a review of fixation and transformation of carbon dioxide. Energy Environ Sci 3:43–81
Liu X-M, Lu GQ, Yan Z-F, Beltramini J (2003) Recent advances in catalysts for methanol synthesis via hydrogenation of CO and CO2. Ind Eng Chem Res 42:6518–6530
Arena F, Italiano G, Barbera K, Bordiga S, Bonura G, Spadaro L, Frusteri F (2008) Solid-state interactions, adsorption sites and functionality of Cu-ZnO/ZrO2 catalysts in the CO2 hydrogenation to CH3OH. Appl Catal A: Gen 350:16–23
Saito M, Fujitani T, Takeuchi M, Watanabe T (1996) Development of copper/zinc oxide-based multicomponent catalysts for methanol synthesis from carbon dioxide and hydrogen. Appl Catal A: Gen 138:311–318
Behrens M, Studt F, Kasatkin I et al (2012) The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 336:893–897
Graciani J, Mudiyanselage K, Xu F et al (2014) Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science 345:546–550
Tisseraud C, Comminges C, Pronier S, Pouilloux Y, Le Valant A (2016) The Cu−ZnO synergy in methanol synthesis part 3: impact of the composition of a selective Cu@ZnOx core−shell catalyst on methanol rate explained by experimental studies and a concentric spheres model. J Catal 343:106–114
Li MM-J, Zeng Z, Liao F, Hong X, Tsang SCE (2016) Enhanced CO2 hydrogenation to methanol over CuZn nanoalloy in Ga modified Cu/ZnO catalysts. J Catal 343:157–167
Gaikwad R, Bansode A, Urakawa A (2016) High-pressure advantages in stoichiometric hydrogenation of carbon dioxide to methanol. J Catal 343:127–132
Arena F, Mezzatesta G, Zafarana G, Trunfio G, Frusteri F, Spadaro L (2013) Effects of oxide carriers on surface functionality and process performance of the Cu−ZnO system in the synthesis of methanol via CO2 hydrogenation. J Catal 300:141–151
Lin S, Ma J, Ye X, Xie D, Guo H (2013) CO hydrogenation on Pd(111): competition between Fischer–Tropsch and oxygenate synthesis pathways. J Phys Chem C 117:14667–14676
Ye J, Liu C-J, Mei D, Ge Q (2014) Methanol synthesis from CO2 hydrogenation over a Pd4/In2O3 model catalyst: a combined DFT and kinetic study. J Catal 317:44–53
Toyir J, de la Piscina PR, Fierro JLG, Homs N (2001) Catalytic performance for CO2 conversion to methanol of gallium-promoted copper-based catalysts: influence of metallic precursors. Appl Catal B: Environ 34:255–266
Nerlov J, Sckerl S, Wambach J, Chorkendorff I (2000) Methanol synthesis from CO2, CO and H2 over Cu(100) and Cu(100) modified by Ni and Co. Appl Catal A: Gen 191:97–109
Jiang X, Koizumi N, Guo X, Song C (2015) Bimetallic Pd−Cu catalysts for selective CO2 hydrogenation to methanol. Appl Catal B: Environ 170:173–185
Wang F, Ding X, Niu X, Liu X, Wang W, Zhang J (2020) Green preparation of core–shell Cu@Pd nanoparticles with chitosan for glucose detection. Carbohydr Polym 247:116647
Gajjala RKR, Palathedath SK (2018) Cu@Pd core-shell nanostructures for highly sensitive and selective amperometric analysis of histamine. Biosens Bioelectron 102:242–246
Chen Y, Yang Y, Fu G, Xu L, Sun D, Lee J-M, Tang Y (2018) Core–shell CuPd@Pd tetrahedra with concave structures and Pd-enriched surface boost formic acid oxidation. J Mater Chem A 6:10632–10638
Li S, Cheng D, Qiu X, Cao D (2014) Synthesis of Cu@Pd core-shell nanowires with enhanced activity and stability for formic acid oxidation. Electrochim Acta 143:44–48
Delley B (2000) From molecules to solids with the DMol3 approach. J Chem Phys 113:7756–7764
Delley B (1990) An all-electron numerical method for solving the local density functional for polyatomic molecules. J Chem Phys 92:508–517
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Perdew JP, Wang Y (1992) Accurate and simple analytic representation of the electron-gas correlation energy. Phys Rev B 45:13244–13249
Bergner A, Dolg M, Küchle W, Stoll H, Preuß H (1993) Ab initio energy-adjusted pseudopotentials for elements of groups 13–17. Mol Phys 80:1431–1441
Halgren TA, Lipscomb WN (1977) The synchronous-transit method for determining reaction pathways and locating molecular transition states. Chem Phys Lett 49:225–232
Govind N, Petersen M, Fitzgerald G, King-Smith D, Andzelm JA (2003) Generalized synchronous transit method for transition state location. Comp Mater Sci 28:250–258
Pan Y, Liu C-J, Ge Q (2008) Adsorption and protonation of CO2 on partially hydroxylated γ-Al2O3 surfaces: a density functional theory study. Langmuir 24:12410–12419
Liu L, Yao H, Jiang Z, Fang T (2018) Theoretical study of methanol synthesis from CO2 hydrogenation on PdCu3(111) surface. Appl Surf Sci 451:333–345
Zhao Y-F, Yang Y, Mims C, Peden CHF, Li J, Mei D (2011) Insight into methanol synthesis from CO2 hydrogenation on Cu(111): complex reaction network and the effects of H2O. J Catal 281:199–221
Peng G, Sibener SJ, Schatz GC, Ceyer ST, Mavrikakis M (2012) CO2 hydrogenation to formic acid on Ni(111). J Phys Chem C 116:3001–3006
Zhang R, Wang B, Liu H, Ling L (2011) Effect of surface hydroxyls on CO2 hydrogenation over Cu/γ-Al2O3 catalyst: a theoretical study. J Phys Chem C 115:19811–19818
Rasmussen PB, Holmblad PM, Askgaard T, Ovesen CV, Stoltze P, Nørskov JK, Chorkendorff I (1994) Methanol synthesis on Cu(100) from a binary gas mixture of CO2 and H2. Catal Lett 26:373–381
Kattel S, Ramírez PJ, Chen JG, Rodriguez JA, Liu P (2017) Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 355:1296–1299
Kattel S, Yan B, Yang Y, Chen JG, Liu P (2016) Optimizing binding energies of key intermediates for CO2 Hydrogenation to methanol over oxide-supported copper. J Am Chem Soc 138:12440–12450
Grabow LC, Mavrikakis M (2011) Mechanism of methanol synthesis on Cu through CO2 and CO hydrogenation. ACS Catal 1:365–384
Christmann K, Demuth JE (1982) The adsorption and reaction of methanol on Pd(100). I. Chem Conden J Chem Phys 76:6308–6317
Yang Y, Evans J, Rodriguez JA, White MG, Liu P (2010) Fundamental studies of methanol synthesis from CO2 hydrogenation on Cu(111), Cu clusters, and Cu/ZnO(0001). Phys Chem Chem Phys 12:9909–9917
Zhang X, Liu J-X, Zijlstra B, Filot IAW, Zhou Z, Sun S, Hensen EJM (2018) Optimum Cu nanoparticle catalysts for CO2 hydrogenation towards methanol. Nano Energy 43:200–209
Acknowledgement
This work is supported by the Applied Basic Research Project of Science and Technology Department of Sichuan Province (No. 2020YJ0418) and the Youth Science and Technology Innovation Team of Southwest Petroleum University (No. 2018CXTD05). We acknowledge the National Supercomputing Center in Shenzhen for providing the computational resources and Materials Studio software.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Yaroslava Yingling.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, J., Ke, Q. & Chen, X. First-principles investigation of methanol synthesis from CO2 hydrogenation on Cu@Pd core–shell surface. J Mater Sci 56, 3790–3803 (2021). https://doi.org/10.1007/s10853-020-05335-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-05335-6