Abstract
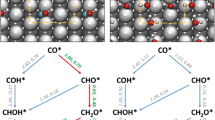
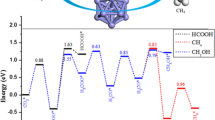
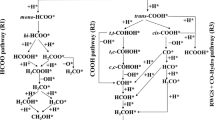
Copper-based catalysts have been widely used for CO2 synthesizing of methanol, while enhancing the productivity of methanol is a big challenge. Here, we chose Co as the partner of Cu, designed an alloyed Co single-atom catalyst (SAC), and calculated its catalytic performance for the hydrogenation of CO2 to CH3OH using density functional theory. Potential energy surface analysis confirmed that the favorable hydrogenation catalyst for CO2 is the SAC of Cu12Co and proceeds via CO2 → HCOO → H2COO → H2CO → H3CO → CH3OH. It has long been proposed that gas-phase atomic clusters that can be well characterized by computational method are the ultimate single-site catalysts. Brønsted–Evans–Polanyi (BEP) relations perform adequately for exploring biomass-relevant chemical kinetics on metal surface with higher accuracy than the universal BEP relations. After BEP relation analysis, the C–H formation and C–O bond scission have shown good correlation within the range considered, and for O–H, the state of initial representation seems more than adequate. We hope that our work may be useful for designing and optimizing Cu-based catalysts for CO2 synthesizing of methanol.







Similar content being viewed by others
References
Schaefer M, Behrendt F, Hammer T (2010) Chem Eng China 4:172–183
Darensbourg D (2010) Inorg Chem 49:10765–10780
Karp EM, Silbaugh TL, Crowe MC, Campbell CT (2012) J Am Chem Soc 134:20388–20395
Lin S, Johnson RS, Smith GK, Xie D, Guo H (2011) Phys Chem Chem Phys 13:9622–9631
Graciani J, Mudiyanselage K, Xu F (2014) Science 345:546–550
Rasul S, Anjum DH, Jedidi A, Minenkov Y, Cavallo L, Takanabe K (2015) Angew Chem Int Ed 54:2146–2153
Porosoff MD, Yan B, Chen JG (2016) Energy Environ Sci 9:62–73
Waugh KC (1992) Catal Lett 15:51–75
Behrens M, Studt F, Kasatkin I et al (2012) Science 336:893–897
Kuld S, Conradsen C, Moses PG et al (2014) Angew Chem 53:5941–5945
Jadhav SG, Vaidya PD, Bhanage BM et al (2014) Chem Eng Res Des 92:2557–2567
Lunkenbein T, Schumann J, Behrens M et al (2015) Angew Chem 54:4544–4548
Kauffman DR, Alfonso D, Matranga C et al (2012) J Am Chem Soc 134:10237–10243
Reske R, Mistry H, Behafarid F et al (2014) J Am Chem Soc 136:6978–6986
Duan X, Warschkow O, Soon A, Delley B, Stampfl C (2010) Matter Mater Phys 81:4764–4770
Liem SY, Clarke JHR, Kresse G (2000) Comput Mater Sci 17:133–140
Mehmood F, Greeley J, Zapol P, Curtiss L (2010) J Phys Chem 114:14458–14466
Sakong S, Groß A (2005) J Catal 231:420–429
Zuo ZJ, Wang L, Han PD, Huang W (2014) Comput Theor Chem 1033:14–22
Natesakhawat S, Lekse JW, Baltrus JP, Ohodnicki PR, Howard BH, Deng XY, Matranga C (2012) ACS Catal 2:1667–1676
Liu C, Yang B, Tyo E, Seifert S, DeBartolo J, von Issendorff B, Zapol P, Vajda S, Curtiss LA (2015) J Am Chem Soc 137:8676–8679
Lei Y, Mehmood F, Lee S, Greeley JP, Lee B, Seifert S, Winans RE, Elam JW, Meyer RJ, Redfern PC et al (2010) Science 328:224–228
Tyo EC, Vajda S (2015) Nat Nanotechnol 10:577–588
Kabir M, Mookerjee A, Bhattacharya AK (2004) Eur Phys J D 31:477–485
Flytzanistephanopoulos M (2014) Acc Chem Res 47:783–792
Liang S, Hao C, Shi Y (2015) Chem Cat Chem 7:2559–2567
Qiu HJ, Ito Y, Cong W et al (2015) Angew Chem 54:14031–14035
Yang S, Kim J, Tak YJ et al (2016) Angew Chem 55:2058–2062
Li Y, Wang Z, Tong X et al (2016) ACS Appl Math Mater Interfaces 28:6959–6965
Lucci FR, Liu J, Marcinkowski MD et al (2015) Nat Commun 6:8550–8558
Catlow CRA, French SA, Sokol AA et al (2005) Philos Trans R Soc Lond A 363:913–936
Morikawa Y, Iwata K, Terakura K (2001) Appl Surf Sci 169:11–15
Yang Y, Evans J, Rodriguez JA et al (2010) Phys Chem Chem Phys PCCP 12:9909–9917
Yang Y, White MG, Ping LA (2011) J Phys Chem A 116:248–256
Studt F, Abild-Pedersen F, Varley JB et al (2013) Catal Lett 143:71–73
Samson K, Śliwa M, Socha RP et al (2014) ACS Catal 4:3730–3741
Kim Y, Trung TSB, Yang S et al (2016) ACS Catal 6:1037–1044
Martínezsuárez L, Siemer N, Frenzel J et al (2015) ACS Catal 5:4201–4218
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865–3868
Hay PJ, Wadt WR (1985) J Chem Phys 82:299–310
Halgren TA, Lipscomb WN (1997) Chem Phys Lett 49:225–232
Nakano H, Nakamura I, Fujitani T, Nakamura J (2001) J Phys Chem B 105:1355–1365
Gokhale AA, Dumesic JA, Mavrikakis MJ (2008) Am Chem Soc 130:1402–1414
Kozuch S, Shaik S (2010) Acc Chem Res 44:101–110
Deng Z, Lu X, Wen Z, Wei S, Zhu Q, Jin D, Shi X, Guo W (2014) RSC Adv 4:12266–12274
Nørskov JK, Bligaard T, Logadottir A, Bahn S, Hansen LB, Bollinger M, Bengaard H, Hammer B, Sljivancanin Z, Mavrikakis M, Xu Y, Dahl S, Jacobsen C (2002) J Catal 209:275–278
Hammer B (1999) Phys Rev Lett 83:3681–3684
Acknowledgements
This work was financially supported by the “1331” project of Shanxi Province, High School 131 Leading Talent Project of Shanxi, Undergraduate Training Programs for Innovation and Entrepreneurship of Shanxi Province (Grant Nos. 105088, 2015537, WL2015CXCY-SJ-01) and Shanxi Normal University (WL2015CXCY-YJ-18) and Teaching Reform Project of Shanxi Normal University (WL2015JGXM-YJ-13) and funded by Graduate student innovation project of Shanxi Normal University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xing, M., Guo, S. & Guo, L. DFT comparison of the performance of bare Cu and Cu-alloyed Co single-atom catalyst for CO2 synthesizing of methanol. Theor Chem Acc 137, 18 (2018). https://doi.org/10.1007/s00214-018-2196-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-018-2196-1