Abstract
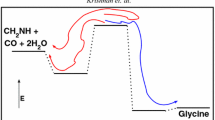
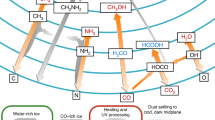
Calculations related to two simple two-step paths (path-I: \( {\mathrm{H}}_2\mathrm{C}=\mathrm{O}+\mathrm{N}{\mathrm{H}}_3\to \upalpha -\mathrm{hydroxy}\ \mathrm{amine}\ \overset{+\mathrm{CO}}{\to }\ \mathrm{glycine}, \) path-II: \( {\mathrm{H}}_2\mathrm{C}=\mathrm{NH}+{\mathrm{H}}_2\mathrm{O}\to \upalpha -\mathrm{hydroxy}\ \mathrm{amine}\ \overset{+\mathrm{CO}}{\to }\ \mathrm{glycine} \)) for the formation of glycine have been discussed. Calculations show that at interstellar conditions these two paths are feasible only in hot cores, not in the cold interstellar clouds (cold core formation is possible only if CH2 = NH, H2O (excess) and CO of path-II, react in a concerted manner). For the laboratory synthesis of glycine, the possibility suggested is via path-I and the reaction being carried out as controlled temperature one-pot synthesis. This study can also be extended to other α-amino acids and possibly enantiomeric excess can be expected. We think this work will not only be able to enrich our future understanding about the formation of amino acids in interstellar medium but also be able to suggest alternative paths for laboratory synthesis of amino acids using either Strecker’s or Miller’s ingredients.

Using computational calculations, two different reaction paths which go through a hemiaminal (α-hydroxyamine) intermediate have been proposed. It has been proposed that the reaction \( {\mathrm{H}}_2\mathrm{C}=\mathrm{O}+\mathrm{N}{\mathrm{H}}_3\to \upalpha -\mathrm{hydroxyamine}\ \overset{+\mathrm{CO}}{\to }\ \mathrm{glycine}, \) is a thermodynamically favorable reaction path in the laboratory conditions, if carried out as a controlled temperature one-pot synthesis. On the hand, it has been argued that the reaction\( {\mathrm{H}}_2\mathrm{C}=\mathrm{NH}+{\mathrm{H}}_2\mathrm{O}\to \upalpha -\mathrm{hydroxy}\ \mathrm{amine}\ \overset{+\mathrm{CO}}{\to }\ \mathrm{glycine} \) is a feasible reaction path in the interstellar conditions, if it proceeds not via the hemiaminal route, rather in a concerted reaction path.






Similar content being viewed by others
References
Ruiz-Mirazo K, Briones C, de la Escosura A (2014) Prebiotic systems chemistry: new perspectives for the origins of life. Chem. Rev. 114:285–366
Herbst E (2001) The chemistry of interstellar space. Chem. Soc. Rev. 30:168–176
Kaiser RI (2002) Experimental investigation on the formation of carbon-bearing molecules in the interstellar medium via neutral−neutral reactions. Chem. Rev. 102:1309–1358
Brown RD, Godfrey PD, Storey JWV, Bassez MP (1978) Microwave-spectrum and conformation of glycine. J. Chem. Soc. Chem. Commun.:547–548
Kuan Y-J, Charnley SB, Huang H–C, Tseng W–L, Kisiel Z (2003) Interstellar Glycine. Astrophys. J. 593: 848–867
Hollis JM, Pedelty JA, Snyder LE, Jewell PR, Lovas FJ, Palmer P, Liu S.–Y (2003) A sensitive very large array search for small-scale glycine emission toward OMC-1. Astrophys. J. 588: 353–359
Snyder LE, Lovas FJ, Hollis JM, Friedel DN, Jewell PR, Remijan A, Ilyushin VV, Alekseev EA, Dyubko SF (2005) A rigorous attempt to verify interstellar glycine. Astrophys. J. 619:914–930
Pizzarello S (2006) The chemistry of life’s origin: a carbonaceous meteorite perspective. Acc. Chem. Res. 39:231–237
Burton AS, Stern JC, Elsila JE, Galvin DP, Dworkin JP (2012) Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem. Soc. Rev. 41:5459–5472
Schmitt-Kopplin P, Gabelica Z, Gougeon RD, Fekete A, Kanawati B, Harir M, Gebefuegi I, Eckel G, Hertkorn N (2010) High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc. Natl. Acad. Sci. 107:2763–2768
Cronin JR, Pizarello S (1997) Enantiomeric excesses in meteoritic amino acids. Science 275:951–955
Engel MH, Nagy B (1982) Distribution and enantiomeric composition of amino acids in the Murchison meteorite. Nature 296:837–840
Kvenvolden K, Lawless J, Pering K, Peterson E, Flores J, Ponnamperuma C, Kaplan IR, Moore C (1970) Evidence for extraterrestrial amino-acids and hydrocarbons in the Murchison meteorite. Nature 228:923–926
Engel MH, Macko SA, Silfer JA (1990) Carbon isotope composition of individual amino acids in the Murchison meteorite. Nature 348:47–49
Callahan MP, Burton AS, Elsila JE, Baker EM, Smith KE, Glavin DP, Dworkin JP (2013) A search for amino acids and nucleobases in the Martian meteorite Roberts Massif 04262 using liquid chromatography-mass spectrometry. Meteorit. Planet. Sci.:48: 786–48: 795
Brown PG, Hildebrand AR, Zolensky MF, Grady M, Clayton RN, Mayeda TK, Tagliaferri E, Spalding R, MacRae ND, Hoffman EJ, Mittlefehldt DW, Wacker JF, Andrew Bird J, Campbell MD, Carpenter R, Gingerich H, Glatiotis M, Greiner E, Mazur MJ, McCausland PJA, Plotkin H, Mazur TR (2000) The fall, recovery, orbit, and composition of the Tagish Lake meteorite: a new type of carbonaceous chondrite. Science 290:320–325
Hiroi T, Zolensky ME, Pieters CM (2001) The Tagish Lake meteorite: a possible sample from a D-type asteroid. Science 293:2234–2236
Lawless JG, Kvenvolden KA, Peterson E, Ponnamperuma C, Moore C (1971) Amino acids indigenous to the Murray meteorite. Science 173:626–627
Elsila JE, Glavin D, Dworkin JP (2009) Cometary glycine detected in samples returned by Stardust. Meteorit. Planet. Sci. 44:1323–1330
Strecker A (1850) Ueber die künstliche Bildung der Milchsäure und einen neuen, dem Glycocoll homologen Körper. Annalen der Chemie und Pharmazie 75:27–45
Strecker A (1854) Ueber einen neuen aus Aldehyd – Ammoniak und Blausäure entstehenden Körper (p ). Annalen der Chemie und Pharmazie 91:349–351
Miller SL (1953) Production of amino acids under possible primitive earth conditions. Science 117:528–529
Miller SL, Urey HC (1959) Organic compound synthesis on the primitive earth. Science 13:245–251
Bada JL (2013) New insights into prebiotic chemistry from Stanley Miller’s spark discharge experiments. Chem. Soc. Rev. 42:2186–2196
Wang L–P, Titov A, McGibbon R, Liu F, Pande VS, Martínez TJ (2014) Discovering chemistry with an ab initio nanoreactor. Nat. Chem. 6: 1044–1048
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian, Inc., Wallingford CT, USA
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98: 5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37:785–789
Curtiss LA, Raghavachari K, Redfern PC, Rassolov V, Pople JA (1998) Gaussian-3 (G3) theory for molecules containing first and second-row atoms. J. Chem. Phys. 109:7764–7776
Baboul AG, Curtiss LA, Redfern PC, Raghavachari K (1999) Gaussian-3 theory using density functional geometries and zero-point energies. J. Chem. Phys. 110:7650–7657
Curtiss LA, Redfern PC, Raghavachari K, Rassolov V, Pople JA (1999) Gaussian-3 theory using reduced Møller-Plesset order. J. Chem. Phys. 110:4703–4709
Curtiss LA, Redfern PC, Raghavachari K (2007) Gaussian-4 theory using reduced order perturbation theory. J. Chem. Phys. 127:124105
Hratchian HP, Schlegel HB (2004) Accurate reaction paths using a Hessian based predictor-corrector integrator. J. Chem. Phys. 120:9918–9924
Ospina E, Villaveces JL (1993) Theoretical calculation of the reaction mechanism between ammonia and formaldehyde. J Mol Struct:THEOCHEM 287:201–209
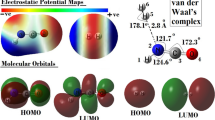
Bhasi P, Nhlabatsi ZP, Sitha S (2016) Expanding the applicability of electrostatic potentials to the realm of transition states. Phys. Chem. Chem. Phys. 18:13002–13009
Shannon RJ, Blitz MA, Goddard A, Heard DE (2013) Accelerated chemistry in the reaction between the hydroxyl radical and methanol at interstellar temperatures facilitated by tunnelling. Nat. Chem. 5:745–749
Smith IWM, Ravishankara AR (2002) Role of hydrogen-bonded intermediates in the bimolecular reactions of the hydroxyl radical. J. Phys. Chem. A 106:4798–4807
Nhlabatsi ZP, Bhasi P, Sitha S (2016) Possible interstellar formation of glycine through a concerted mechanism: a computational study on the reaction of CH2=NH, CO2 and H2. Phys. Chem. Chem. Phys. 18:20109–20117
Pal R, Nagendra G, Samarasimhareddy M, Sureshbabu VV, Guru Row TN (2015) Observation of a reversible isomorphous phase transition and an interplay of “σ-holes” and “π-holes” in Fmoc-Leu-ψ[CH2-NCS]. Chem. Commun. 51:933
Solimannejad M, Ramezani V, Trujillo C, Alkorta I, Sanchez G, Elguero J (2012) Competition and interplay between σ-hole and π-hole interactions: a computational study of 1:1 and 1:2 complexes of nitryl halides (O2NX) with ammonia. J. Phys. Chem. A 116:5199
Bauza A, Mooibroek TJ, Frontera A (2015) The bright future of unconventional σ/π-hole interactions. Chemphyschem 16:2496
Murray JS, Lane P, Clark T, Riley KE, Politzer P (2012) Σ-holes, π-holes and electrostatically-driven interactions. J. Mol. Model. 18:541–548
Baymak MS, Zuman P (2007) Equilibria of formation and dehydration of the carbinolamine intermediate in the reaction of benzaldehyde with hydrazine. Tetrahedron 63:5450–5454
Evans DA, Borg G, Scheidt KA (2002) Remarkably stable tetrahedral intermediates: carbinols from nucleophilic additions to N-acylpyrroles. Angew. Chem. Int. Ed. 41:3188–3191
Hooley RJ, Iwasawa T, Rebek Jr J (2007) Detection of reactive tetrahedral intermediates in a deep cavitand with an introverted functionality. J. Am. Chem. Soc. 129:15330–15339
Iwasawa T, Hooley RJ, Rebek Jr J (2007) Stabilization of labile carbonyl addition intermediates by a synthetic receptor. Science 317:493–496
Xu L, Hua S, Li S (2013) Insight into the reaction between a primary amine and a cavitand with an introverted aldehyde group: an enzyme-like mechanism. Chem. Commun. 49:1542–1544
Kawamichi T, Haneda T, Kawano M, Fujita M (2009) X-ray observation of a transient hemiaminal trapped in a porous network. Nature 461:633–635
Morris W, Doonan CJ, Yaghi OM (2011) Postsynthetic modification of a metal-organic framework for stabilization of a hemiaminal and ammonia uptake. Inorg. Chem. 50:6853–6855
Dolotko O, Wiench JW, Dennis KW, Pecharsky VK, Balema VP (2010) Mechanically induced reactions in organic solids: liquid eutectics or solid-state processes? New J. Chem. 34:25–28
For a complete up-to-date list of interstellar and circumstellar molecules, see http://www.astrochymist.org/astrochymist_ism.html and http://www.astro.uni-koeln.de/cdms/molecules. (Webpages were last accessed on 22ND July 2019)
Tennyson J (2003) “Molecules in space” in “Handbook of Molecular Physics and Quantum Chemistry” Eds. Wilson S 3:356–369
Menten KM, Wyrowski F (2011) Molecules detected in interstellar space in “Interstellar Molecules”, 241: 27-42
Yamada KMT, Winnewisser G (2011) List of molecules observed in interstellar space in “Interstellar Molecules”, 241: 219-223
Müller HSP, Schlöder F, Thorwirth S, Winnewisser G (2004) The cologne database for molecular spectroscopy, CDMS in “The Dense Interstellar Medium in Galaxies”, 91: 95-98
Snyder LE, Buhl D, Zuckerman B, Palmer P (1969) Microwave detection of interstellar formaldehyde. Phys. Rev. Lett. 22:679–681
Nguyen-Q-Rieu GD, Bujarrabal V (1984) Ammonia and cyanotriacetylene in the envelopes of CRL 2688 and IRC + 10216. Astron. Astrophys. 138:L5–L8
Ziurys LM (2006) The chemistry in circumstellar envelopes of evolved stars: following the origin of the elements to the origin of life. Proc. Natl. Acad. Sci. U. S. A. 103:12274–12279
Godfrey PD, Brown RD, Robinson BJ, Sinclair MW (1973) Discovery of interstellar methanimine (formaldimine). Astrophys Lett 13:119–121
Dickens JE, Irvine WM, DeVries CH, Ohishi M (1997) Hydrogenation of interstellar molecules: a survey for methylenimine (CH2NH). Astrophys. J. 479:307–312
Salter CJ, Ghosh T, Catinella B, Lebron M, Lerner MS, Minchin R, Momjian E (2008) The arecibo ARP 220 spectral census. I. Discovery of the pre-biotic molecule methanimine and new cm-wavelength transitions of other molecules. Astron. J. 136:389–399
Dyson JE, Williams DA (1997) The physics of the interstellar medium. Series in astronomy and astrophysics2nd edn. Bristol, Institute of Physics
Garrod RT, Weaver SLW (2013) Simulations of hot-core chemistry. Chem. Rev. 113:8939–8960
van Dishoeck EF, Blake GA (1998) Chemical evolution of star-forming regions. Ann Rev Astron Astrophys 36:317–368
http://astronomy.swin.edu.au/cosmos/I/interstellar+gas+cloud. (Webpage was last accessed on 22nd July 2019)
Nhlabatsi ZP, Bhasi P, Sitha S (2016) Possible interstellar formation of glycine from the reaction of CH2=NH, CO and H2O: catalysis by extra water molecules through the hydrogen relay transport. Phys. Chem. Chem. Phys. 18:375–381
Bhasi P, Nhlabatsi ZP, Sitha S (2015) Reaction between HN and SN: a possible channel for the interstellar formation of N2 and SH in the cold interstellar clouds. Phys. Chem. Chem. Phys. 17:32455–32463
Acknowledgments
The authors like to thank University of Johannesburg for support. ZPN and PB carried out this work while doing their PhD works at University of Johannesburg.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Energetics data related to the potential energy surfaces for the reactions 1–3, calculated using various methods and basis sets (discussed in the computational section) are tabulated in Table S1 and S2. Also provided with are the optimized Cartesian coordinates of all the stationary points.
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Nhlabatsi, Z.P., Bhasi, P. & Sitha, S. Hemiaminal route for the formation of interstellar glycine: a computational study. J Mol Model 25, 335 (2019). https://doi.org/10.1007/s00894-019-4224-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4224-z