Abstract
In this paper, we perform the synthesization of carbon nanoparticles for active principle vectorization, with the suggestion of a reaction mechanism of tryptophan methyl ester addition on [60]fullerene. Firstly, we studied the effect of tryptophan form on its addition reaction on [60]fullerene. So, in order to determine the preferred environment that makes this reaction the most favorable, we considered all tryptophan possible forms in our investigation: the molecular, the zwitterionic, and the dibasic forms. Secondly, we investigate the proposed reaction mechanism of tryptophan methyl ester addition on [60]fullerene using theoretical thermodynamic calculation. Our hypothesis suggests the formation of azomethine ylide molecule in a first step followed by its addition on [60]fullerene in the second step by the photo-addition reaction involving the oxygen in its singlet state. The stability of each reactive intermediate involved in this mechanism is verified thermodynamically. The 12 most stable conformations of azomethine ylide were observed through potential energy surface analysis. They were obtained by a relaxed scan of the four dihedral angles. The calculations were conducted on the optimized geometry of fulleropyrrolidine mono-adduct and the bulk values of its thermodynamic constants were also determined. Infrared spectra observed in 100–4000 cm−1 region confirmed our hypothesis suggesting the first step of azomethine ylide formation followed by the second step of azomethine ylide addition on [60]fullerene by ν(Caliphatic-C-N), ν(Caromatic-C-N) and δ(N-H) coupled with ν(C-N) absorption bond.

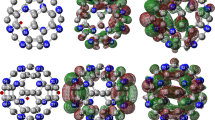
Optimized geometry of the Fulleropyrrolidine monoaduct molecule.







Similar content being viewed by others
References
Brack A, Raulin F (1991) L’évolution chimique et les origines de la vie. Masson, Paris
Friedman M (1999) Chemistry, nutrition, and microbiology of d-amino acids. J Agric Food Chem 47:3457–3479
Rosset R, Caude M, Jardy A (1991) Chromatographies en Phases liquide et Supercritique. Masson, Paris
Jing T, Li X-Y (2002) Ab initio study on electron excitation and electron transfer in tryptophan–tyrosine system. Chem Phys 284:543–554
Galikova N, Kelminskas M, Gruodis A, Balevichius LM (2011) Tyrosine-tryptophan complex: intermolecular electron transfer using quantum chemistry approach. Comput Model New Technol 15:19–23
Omri N, Yahyaoui M, Banani R, Messaoudi S, Moussa F, Abderrabba M (2016) Ab-initio HF and density functional theory investigations on the synthesis mechanism, conformational stability, molecular structure and UV spectrum of N′-formylkynurenine. J Theor Comput Chem 15:1650006–1650022
Schettino V, Pagliai M, Ciabini L, Cardini G (2001) The vibrational spectrum of fullerene C60. J Phys Chem A 105:11192–11196
Loboda O, Zales’ny R, Avramopoulos A, Luis JM, Kirtman B, Tagmatarchis N, Reis H, Papadopoulos MG (2009) Linear and nonlinear optical properties of [60]fullerene derivatives. J Phys Chem A 113:1159–1170
Farré M, Pérez S, Gajda-Schrantz K, Osorio V, Kantiani L, Ginebreda A, Barceló D (2010) First determination of C60 and C70 fullerenes and N-methylfulleropyrrolidine C60 on the suspended material of wastewater effluents by liquid chromatography hybrid quadrupole linear ion trap tandem mass spectrometry. J Hydrol 383:44–51
Isaacson CW, Bouchard D (2010) Asymmetric flow field flow fractionation of aqueous C60 nanoparticles with size determination by dynamic light scattering and quantification by liquid chromatography atmospheric pressure photo-ionization mass spectrometry. J Chromatogr A 1217:1506–1512
Romanova VS, Tsyryapkin VA, Lyakhovetsky YI, Parnes ZN, Vol’pin ME (1994) Addition of amino acids and dipeptides to fullerene C60 giving rise to monoadducts. Russ Chem Bull 43:1090–1091
Zhou DJ, Gan LB, Xu LB, Luo CP, Huang CH (1995) Glycine C60 adduct and its rare earth complexes. Fuller Sci Technol 3:127–131
Bianco A, Da Ros T, Prato M, Toniolo C (2001) Fullerene-based amino acids and peptides. J Pept Sci 7:208–219
An YZ, Anderson JL, Rubin Y (1993) Synthesis of alpha-amino acid derivatives of C60 from 1, 9-(4-hydroxycyclohexano) buckminsterfullerene. J Org Chem 58:4799–4799
Ohno M, Kojima S, Shirakawa Y, Eguchi S (1997) Fusion of C60 with cyclic amino acid and Thiourea by hetero Diels-Alder reactions. Heterocycles 46:49–52
Burley GA, Keller PA, Pyne SG (1999) [60]fullerene amino acids and related derivatives. Fuller Sci Technol 7:973–1001
Krusic PJ (1991) Radical reactions of C60. Science 254:1183–1188
Jensen AW, Wilson SR, Schuster DI (1996) Biological applications of fullerenes. Bioorg Med Chem 190-192:199–207
Skanji R, Messaouda MB, Zhang Y, Abderrabba M, Szwarc H, Moussa F (2012) Sequential photo-addition of glycine methyl-ester to [60]fullerene. Tetrahedron 63:2713–2718
Prato P, Chan Li Q, Wudl F, Lucchini V (1993) Addition of azides to fullerene C60: synthesis of azafulleroids. J Am Chem Soc 115:1148–1150
Troshin PA, Kornev AB, Peregudov SM, Lyubovskaya RN (2007) Benzylamine imines as versatile precursors to azomethine and nitrile ylides in the [2 + 3] cycloaddition reactions with [60]fullerene. Mendeleev Commun 17:116–118
Maggini M, Scorrano G, Prato M (1993) Addition of azomethine ylides to C60: synthesis, characterization, and functionalization of fullerene pyrrolidines. J Am Chem Soc 115:9798–9799
Khemiri N, Messaoudi S, Moussa F, Abderrabba M, Chermette H (2016) Theoretical investigation on two different mechanisms of fulleropyrrolidine formation. Theor Chem Acc 135:265–274
Omri N, Yahyaoui M, Banani R, Messaoudi S, Moussa F, Abderrabba M (2016) Density functional theory investigation on the conformational analysis, molecular structure and FT-IR spectra of tryptophan methyl-ester molecule. Int J Innov Appl Stud 17:13–32
Lakowicz JR (1999) Pricipales of fluorescence spectroscopy. Kluwer Academic/Plenum Publishers, New York
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA et al (2009) Gaussian 09 revision A02. Gaussian Inc., Wallingford
Frisch A, Neilson AB, Holder AJ (2009) GAUSSVIEW, user manual. Gaussian Inc., Pittsburgh
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Perdew JP, Burke K, Wang Y (1996) Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys Rev B 54:16533–16539
Vosko SH, Wilk L, Nusair M (1980) Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can J Phys 58:1200–1211
Michael CM, Du X, Kwon J, Mihaly L (1994) Observation and assignment of silent and higher-order vibrations in the infrared transmission of Csu crystals. Phys Rev B 50:173–180
Choi CH, Kertesz M, Mihaly L (2000) Vibrational assignment of all 46 fundamentals of C60 and C60 6−: scaled quantum mechanical results performed in redundant internal coordinates and compared to experiments. J Phys Chem A 104:102–108
Schettino V, Salvi PR, Bini R, Cardini G (1994) On the vibrational assignment of fullerene C60. J Chem Phys 101:11079–11081
Giannozzi P, Baroni S (1994) Vibrational and dielectric properties of C60 from density functional perturbation theory. J Chem Phys 100:8537–8539
Acknowledgements
The authors would like to thank the Laboratory of Materials, Molecules, and Applications (LMMA, Tunis) for the research fellowship and the laboratory of studies of technical and analytical molecular instruments (LETIAM, Paris) for the collaboration.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Omri, N., Khemiri, N., Abderrabba, M. et al. Addition of tryptophan methyl-ester on [60]fullerene: theoretical investigation of the mechanisms of azomethine ylides and fulleropyrrolidine formation. J Mol Model 24, 270 (2018). https://doi.org/10.1007/s00894-018-3760-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3760-2