Abstract
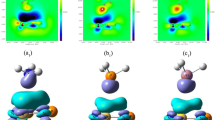
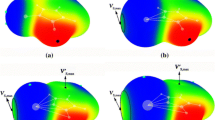
For inorganic benzenes C3N3X3 and B3O3X3 (X = H, F, CN), the positive electrostatic potentials (π-hole) were discovered above and below the inorganic benzene ring center. Then, the π-hole interactions between the inorganic benzenes and NCH have been designed and investigated by MP2/aug-cc-pVDZ calculations. In this paper, the termolecular complexes B3O3X3···NCH···NCH, C3N3X3···NCH···NCH (X = H, F, CN) were also designed to illustrate the enhancing effects of the H···N hydrogen bond on the π-hole interactions. The π-hole interaction energy was influenced by the strength of different electron-withdrawing substituents of inorganic benzenes, gradually increasing in the order of X = H, F, CN. What’s more, the π electron densities account for 71~88% of the total electron densities, indicating the strength of interaction energy is mainly determined by π-type electron densities.

The termolecular complexes B3O3X3···NCH···NCH, C3N3X3···NCH···NCH (X = H, F, CN) were designed to illustrate the enhancing effects of the H···N hydrogen bond on the π-hole interactions.






Similar content being viewed by others
References
Müller-Dethlefs K, Hobza P (2000) Chem Rev 100:143–167
Cavallo G, Metrangolo P, Milani R, Pilati T, Priimagi A, Resnati G, Terraneo G (2016) Chem Rev 116:2478–2601
Auffinger P, Hays FA, Westhof E, Ho PS (2004) Proc Natl Acad Sci U S A 101:16789–16794
Metrangolo P, Resnati G, Pilati T, Biella S (2007) Acta Crystallogr 61:105–136
Clark T, Hennemann M, Murray JS, Politzer P (2007) J Mol Model 13:291–296
Wang H, Wang W, Jin WJ (2016) Chem Rev 116:5072–5104
Murray JS, Concha MC, Lane P, Hobza P, Politzer P (2008) J Mol Model 14:699–704
Murray JS, Lane P, Politzer P (2007) Int J Quantum Chem 107:2286–2292
Murray JS, Lane P, Clark T, Riley KE, Politzer P (2012) J Mol Model 18:541–548
Lang T, Li X, Meng L, Zheng S, Zeng Y (2014) Struct Chem 26:213–221
Murray JS, Shields ZP, Seybold PG, Politzer P (2015) J Comput Sci 10:209–216
Politzer P, Murray JS, Clark T (2010) Phys Chem Chem Phys 12:7748–7757
Politzer P, Murray JS (2017) J Comput Chem. https://doi.org/10.1002/jcc.24891
Zeng Y, Zhang X, Li X, Meng L, Zheng S (2011) ChemPhysChem 12:1080–1087
Pierrefixe SCAH, Bickelhaupt FM (2008) Aust J Chem 61:209–215
Engelberts JJ, Havenith RWA, van Lenthe JH, Jenneskens LW, Fowler PW (2005) Inorg Chem 44:5266–5272
Jemmis ED, Kiran B (1998) Inorg Chem 37:2110–2116
Cyrański MK, Krygowski TM, Katritzky AR, Schleyer PR (2002) J Organomet Chem 67:1333–1338
Cyrański MK, Schleyer PR, Krygowski TM, Jiao H, Hohlneicher G (2003) Tetrahedron 59:1657–1665
Phukan AK, Guha AK, Silvi B (2010) Dalton Trans 39:4126–4137
Wu W, Li X, Meng L, Zheng S, Zeng Y (2015) J Phys Chem A 119:2091–2097
Matsunaga N, Gordon MS (1994) J Am Chem Soc 116:11407–11419
Feng XJ, Zhang M, Zhao LX, Zhang HY, Luo YH (2014) Comput Theor Chem 1029:84–90
Fowler PW, Steiner E (1997) J Phys Chem A 101:1409–1413
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA et al. (2010) Gaussian 09. Gaussian Inc., Wallingford
Møller C, Plesset MS (1934) Phys Rev 46:618–622
Woon DE, Dunning TH (1995) J Phys Chem A 103:4572–4585
Dunning TH (1989) J Phys Chem A 90:1007–1023
Boys SF, Bernardi F (2006) Mol Phys 19:553–566
Politzer P, Truhlar DG (1981) Chemical applications of atomic and molecular electrostatic potentials. Plenum, New York
Bulat FA, Toro-Labbe A, Brinck T, Murray JS, Politzer P (2010) J Mol Model 16:1679–1691
Bader RFW (1990) Atoms in molecules—a quantum theory. Oxford University Press, Oxford
Popelier PLA (2000) Atoms in molecules: an introduction. Pearson, Harlow
Biegler-König F (2000) AIM 2000, version 1.0. University of Applied Science Bielefeld, Germany
Lu T, Chen F (2012) J Comput Chem 33:580–592
Azami SM (2010) J Phys Chem A 114:11794–11797
Zeng Y, Zhu M, Meng L, Zheng S (2011) ChemPhysChem 12:3584–3590
Zeng Y, Wu W, Li X, Zheng S, Meng L (2013) ChemPhysChem 14:1591–1600
Daudel R (1952) C R Acad Sci 235:886–888
Roux M, Besnainou S, Daudel R (1956) J Chim Phys 53:218–221
Roux M, Daudel R (1995) C R Acad Sci 240:90–92
Zheng SJ, Hada M, Nakatsuji H (1996) Theor Chim Acta 93:67–78
Li X, Zeng Y, Zhang X, Zheng S, Meng L (2011) J Mol Model 17:757–767
Politzer P, Riley KE, Bulat FA, Murray JS (2012) Comput Theor Chem 998:2–8
Acknowledgments
This project was supported by the National Natural Science Foundation of China (Contract Nos: 21371045, 21373075), the Natural Science Foundation of Hebei Province (Contract Nos: B2015205045). Thanks are also due to the Education Department of Hebei Province of China through innovative hundred talents support program (SLRC2017041).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The manuscript has full control of all primary data, and the authors agree to allow the journal to review their data if requested. The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Chu, R., Zhang, X., Meng, L. et al. Inorganic benzenes as the noncovalent interaction donor: a study of the π-hole interactions. J Mol Model 23, 335 (2017). https://doi.org/10.1007/s00894-017-3513-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3513-7