Abstract
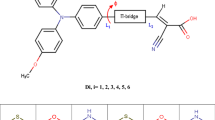
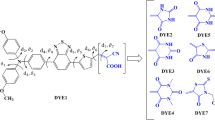
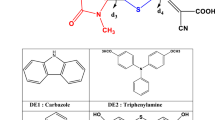
The ground state and excited state properties of three coumarin dyes, ZCJ1, ZCJ2 and ZCJ3, including ground state structures, energy levels, absorption spectra and driving forces of electron injection, were investigated via density functional theory (DFT) and time-dependent density functional theory (TD-DFT). In addition, five new molecules ZCJ3-1, ZCJ3-2, ZCJ3-3, ZCJ3-4 and ZCJ3-5 were designed through the introduction of a –CN group into molecule ZCJ3. The ground state and excited state properties of the five designed molecules were also calculated and compared with that of the original molecule, aiming to investigate the effect of different position of –CN groups on the optical and electrical properties of dye molecules. Moreover, the external electric field was taken into account. The results indicated that all three original molecules have better absorption within the visible-light range, and the molecule with a thiophene–thiophene conjugated bridge enables a red shift of the absorption spectrum. The molecule with a thiophene–benzene ring conjugated bridge enables the increase of driving force of electron injection. The energy levels, spectra and driving force of electron injection for the designed molecules are discussed in terms of studying their potential utility in dye-sensitized solar cells.








Similar content being viewed by others
References
O’Regan B, Gratzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353(6346):737–740
Grätzel M (2009) Recent advances in sensitized mesoscopic solar cells. Acc Chem Res 42(11):1788–1798
Cao Y, Bai Y, Yu Q, Cheng Y, Liu S, Shi D, Gao F, Wang P (2009) Dye-sensitized solar cells with a high absorptivity ruthenium sensitizer featuring a 2-(hexylthio)thiophene conjugated bipyridine. J Phys Chem C 113(15):6290–6297
Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H (2010) Dye-sensitized solar cells. Chem Rev 110(11):6595–6663
Yella A, Lee H-W, Tsao HN, Yi C, Chandiran AK, Nazeeruddin MK, Diau EW-G, Yeh C-Y, Zakeeruddin SM, Grätzel M (2011) Porphyrin-sensitized solar cells with cobalt (II/III)–based redox electrolyte exceed 12 percent efficiency. Science 334(6056):629–634
Kanaparthi RK, Kandhadi J, Giribabu L (2012) Metal-free organic dyes for dye-sensitized solar cells: recent advances. Tetrahedron 68(40):8383–8393
Mishra A, Fischer MKR, Bäuerle P (2009) Metal-free organic dyes for Dye-sensitized solar cells: from structure: property relationships to design rules. Angew Chem Int Ed 48(14):2474–2499
Zhang YH, Ren PH, Li YZ, Su RZ, Zhao MY (2015) Optical absorption and electron injection of 4-(cyanomethyl)benzoic acid based dyes: a DFT study. J Chem 2015:9
Li H, Chen M (2013) Structure–property relationships for three indoline dyes used in dye-sensitized solar cells: TDDFT study of visible absorption and photoinduced charge-transfer processes. J Mol Model 19(12):5317–5325
Wang Z-S, Cui Y, Dan-oh Y, Kasada C, Shinpo A, Hara K (2008) Molecular design of coumarin dyes for stable and efficient organic dye-sensitized solar cells. J Phys Chem C 112(43):17011–17017
Urbani M, Grätzel M, Nazeeruddin MK, Torres T (2014) Meso-substituted porphyrins for dye-sensitized solar cells. Chem Rev 114(24):12330–12396
Mathew S, Iijima H, Toude Y, Umeyama T, Matano Y, Ito S, Tkachenko NV, Lemmetyinen H, Imahori H (2011) Optical, electrochemical, and photovoltaic effects of an electron-withdrawing tetrafluorophenylene bridge in a push–pull porphyrin sensitizer used for dye-sensitized solar cells. J Phys Chem C 115(29):14415–14424
Kajiyama S, Uemura Y, Miura H, Hara K, Koumura N (2012) Organic dyes with oligo-n-hexylthiophene for dye-sensitized solar cells: relation between chemical structure of donor and photovoltaic performance. Dyes Pigments 92(3):1250–1256
Zafer C, Gultekin B, Ozsoy C, Tozlu C, Aydin B, Icli S (2010) Carbazole-based organic dye sensitizers for efficient molecular photovoltaics. Sol Energy Mater Sol Cells 94(4):655–661
Horiuchi T, Miura H, Sumioka K, Uchida S (2004) High efficiency of Dye-sensitized solar cells based on metal-free indoline dyes. J Am Chem Soc 126(39):12218–12219
Ito S, Miura H, Uchida S, Takata M, Sumioka K, Liska P, Comte P, Pechy P, Gratzel M (2008) High-conversion-efficiency organic dye-sensitized solar cells with a novel indoline dye. Chem Commun 44(41):5194–5196
Kim S, Lee JK, Kang SO, Ko J, Yum JH, Fantacci S, De Angelis F, Di Censo D, Nazeeruddin MK, Grätzel M (2006) Molecular engineering of organic sensitizers for solar cell applications. J Am Chem Soc 128(51):16701–16707
Gupta A, Armel V, Xiang W, Bilic A, Evans RA (2015) New organic sensitizers using 4-(cyanomethyl)benzoic acid as an acceptor group for dye-sensitized solar cell applications. Dyes Pigments 113:280–288
Hara K, Tachibana Y, Ohga Y, Shinpo A, Suga S, Sayama K, Sugihara H, Arakawa H (2003) Dye-sensitized nanocrystalline TiO2 solar cells based on novel coumarin dyes. Sol Energy Mater Sol Cells 77(1):89–103
Ying W, Yang J, Wielopolski M, Moehl T, Moser J-E, Comte P, Hua J, Zakeeruddin SM, Tian H, Gratzel M (2014) New pyrido[3,4-b]pyrazine-based sensitizers for efficient and stable dye-sensitized solar cells. Chem Sci 5(1):206–214
Zhong C, Gao J, Cui Y, Li T, Han L (2015) Coumarin-bearing triarylamine sensitizers with high molar extinction coefficient for dye-sensitized solar cells. J Power Sources 273:831–838
Sun M, Xu H (2012) A novel application of plasmonics: plasmon-driven surface-catalyzed reactions. Small 8(18):2777–2786
Zhao G-J, Liu J-Y, Zhou L-C, Han K-L (2007) Site-selective photoinduced electron transfer from alcoholic solvents to the chromophore facilitated by hydrogen bonding: a new fluorescence quenching mechanism. J Phys Chem B 111(30):8940–8945
Zhao G-J, Han K-L (2007) Ultrafast hydrogen bond strengthening of the photoexcited fluorenone in alcohols for facilitating the fluorescence quenching. J Phys Chem A 111(38):9218–9223
Li Y, Pullerits T, Zhao M, Sun M (2011) Theoretical characterization of the PC60BM:PDDTT model for an organic solar cell. J Phys Chem C 115(44):21865–21873
Xie M, Bai F-Q, Wang J, Kong C-P, Chen J, Zhang H-X (2016) Theoretical description of dye regeneration on the TiO2–dye–electrolyte model. Comput Mater Sci 111:239–246
Sun C, Li Y, Qi D, Li H, Song P (2016) Optical and electrical properties of purpurin and alizarin complexone as sensitizers for dye-sensitized solar cells. J Mater Sci Mater Electron 27:8027–8039
Ren X-F, Kang G-J, He Q-Q (2015) Triphenylamine-based indoline derivatives for dye-sensitized solar cells: a density functional theory investigation. J Mol Model 22(1):1–9
Zarate X, Schott-Verdugo S, Rodriguez-Serrano A, Schott E (2016) The nature of the donor motif in acceptor-bridge-donor dyes as an influence in the electron photo-injection mechanism in DSSCs. J Phys Chem A 120(9):1613–1624
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136(3B):B864–B871
Becke AD (1993) Density‐functional thermochemistry. III. The role of exact exchange. J Chem Phys 98(7):5648–5652
Stratmann RE, Scuseria GE, Frisch MJ (1998) An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J Chem Phys 109(19):8218–8224
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange–correlation functional using the coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393(1–3):51–57
Ordon P, Tachibana A (2005) Investigation of the role of the C-PCM solvent effect in reactivity indices. J Chem Sci 117(5):583–589
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd J, Brothers EN, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision A.02. Gaussian, Inc, Wallingford, CT, USA
Song P, Li YZ, Ma FC, Sun MT (2015) Insight into external electric field dependent photoinduced intermolecular charge transport in BHJ solar cell materials. J Mater Chem C 3(18):4810–4819
Li H, Li Y, Chen M (2014) Molecular design of organic sensitizers absorbing over a broadened visible region for dye-sensitized solar cells. RSC Adv 4(101):57916–57922
Li YZ, Sun CF, Qi DW, Song P, Ma FC (2016) Effects of different functional groups on the optical and charge transport properties of copolymers for polymer solar cells. RSC Adv 6:61809–61820
Cui L, Wang P, Fang Y, Li Y, Sun M (2015) A plasmon-driven selective surface catalytic reaction revealed by surface-enhanced Raman scattering in an electrochemical environment. Sci Rep 5:11920
Sun CF, Qi DW, Li YZ, Yang LP (2015) Tunable spectra and charge transfer process of benzodifurandione-based polymer by sulfur substitution. RSC Adv 5(24):18492–18500
Kleinman DA (1962) Nonlinear dielectric polarization in optical media. Phys Rev 126(6):1977–1979
Olbrechts G, Munters T, Clays K, Persoons A, Kim O-K, Choi L-S (1999) High-frequency demodulation of multi-photon fluorescence in hyper-Rayleigh scattering. Opt Mater 12(2–3):221–224
Rehm D, Weller A (1970) Kinetics of fluorescence quenching by electron and H-atom transfer. Isr J Chem 8(2):259–271
Qin C, Clark AE (2007) DFT characterization of the optical and redox properties of natural pigments relevant to dye-sensitized solar cells. Chem Phys Lett 438(1–3):26–30
Hinchliffe A, Soscún HJ (2005) Ab initio studies of the dipole moment and polarizability of azulene in its ground and excited singlet states. Chem Phys Lett 412(4–6):365–368
Acknowledgements
This work was supported by Heilongjiang Postdoctoral Grant (LBH-Z15002), China Postdoctoral Science Foundation (2016 M590270), the National Natural Science Foundation of China (Grant Nos. 11404055 and 11374353), the Fundamental Research Funds for the Central Universities (Grant No. 2572014CB31), the Heilongjiang Provincial Youth Science Foundation (Grant Nos. QC2013C006), National Undergraduate Innovative and Entrepreneurial Training Program (Grant No: 201610225099) and Academic Research Training of NEFU for Undergraduate (Grant No: KY2015020).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, C., Bai, Y., Li, Y. et al. Controlling light absorption and photoelectric properties of coumarin-triphenylaminedye by different acceptor functional groups. J Mol Model 22, 277 (2016). https://doi.org/10.1007/s00894-016-3149-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-3149-z