Abstract
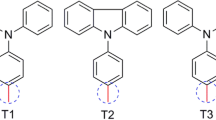
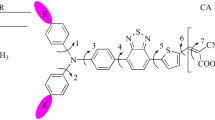
Design and synthesis of new potent sensitizers are of interest for realization of high-efficiency Dye Sensitized Solar Cells (DSSCs). Modification of the triphenylamine-based dyes by introducing suitable anchoring groups aimed at improvement of optoelectronic properties is attempted in our work. The molecular structure, molecular orbitals and energies, electronic absorption spectra, free energies of electron injection and dye regeneration, chemical reactivity parameters and adsorption to TiO2 semiconductor have been reported. Density functional theory (DFT) and time-dependent DFT (TD-DFT) were used to obtain the reported properties. The results reveal superior optical, electronic properties, chemical reactivity parameters and adsorption energies for the investigated dyes. The findings evince that the dyes featuring heterocyclic anchoring groups could be potential candidates for DSSCs’ applications; the new materials are worthy of being investigated experimentally.







Similar content being viewed by others
References
Gong J, Sumathy K, Qiao Q, Zhou Z (2017) Review on dye-sensitized solar cells (DSSCs): advanced techniques and research trends. Renew Sust Energ Rev 68:234–246. https://doi.org/10.1016/j.rser.2016.09.097
Jung HS, Lee J-K (2013) Dye sensitized solar cells for economically viable photovoltaic systems. J Phys Chem Lett 4(10):1682–1693. https://doi.org/10.1021/jz400112n
Hug H, Bader M, Mair P, Glatzel T (2014) Biophotovoltaics: natural pigments in dye-sensitized solar cells. Appl Energy 115:216–225. https://doi.org/10.1016/j.apenergy.2013.10.055
O'Regan B, Grätzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353(6346):737–740. https://doi.org/10.1038/353737a0
Kakiage K, Aoyama Y, Yano T, Oya K, J-i F, Hanaya M (2015) Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem Commun 51(88):15894–15897. https://doi.org/10.1039/C5CC06759F
Ahmad MS, Pandey AK, Rahim NA (2017) Advancements in the development of TiO2 photoanodes and its fabrication methods for dye sensitized solar cell (DSSC) applications. A review. Renew Sust Energ Rev 77:89–108. https://doi.org/10.1016/j.rser.2017.03.129
Han L, Meng X, Ye H, Cui Y (2019) Novel D-π-A benzocarbazole dyes with simple structures for efficient dye-sensitized solar cells. J Photochem Photobiol A Chem 376:127–134. https://doi.org/10.1016/j.jphotochem.2019.03.015
Chen C, Yang X, Cheng M, Zhang F, Sun L (2013) Degradation of cyanoacrylic acid-based organic sensitizers in dye-sensitized solar cells. ChemSusChem 6(7):1270–1275. https://doi.org/10.1002/cssc.201200949
Baik C, Kim D, Kang M-S, Kang SO, Ko J, Nazeeruddin MK, Grätzel M (2009) Organic dyes with a novel anchoring group for dye-sensitized solar cell applications. J Photochem Photobiol A Chem 201(2–3):168–174. https://doi.org/10.1016/j.jphotochem.2008.10.018
Murakami TN, Yoshida E, Koumura N (2014) Carbazole dye with phosphonic acid anchoring groups for long-term heat stability of dye-sensitized solar cells. Electrochim Acta 131:174–183. https://doi.org/10.1016/j.electacta.2013.12.013
Jia H-L, Peng Z-J, Gong B-Q, Huang C-Y, Guan M-Y (2019) New 2D–π–2A organic dyes with bipyridine anchoring groups for DSSCs. New J Chem 43(15):5820–5825. https://doi.org/10.1039/C9NJ00087A
Deogratias G, Seriani N, Pogrebnaya T, Pogrebnoi A (2020) Tuning optoelectronic properties of triphenylamine based dyes through variation of pi-conjugated units and anchoring groups: a DFT/TD-DFT investigation. J Mol Graph Model 94:107480. https://doi.org/10.1016/j.jmgm.2019.107480
Zhang J, Zhu H-C, Zhong R-L, Wang L, Su Z-M (2018) Promising heterocyclic anchoring groups with superior adsorption stability and improved IPCE for high-efficiency noncarboxyl dye sensitized solar cells: a theoretical study. Org Electron 54:104–113. https://doi.org/10.1016/j.orgel.2017.12.023
Hagberg DP, Marinado T, Karlsson KM, Nonomura K, Qin P, Boschloo G, Brinck T, Hagfeldt A, Sun L (2007) Tuning the HOMO and LUMO energy levels of organic chromophores for dye sensitized solar cells. J Org Chem 72(25):9550–9556. https://doi.org/10.1021/jo701592x
Xu M, Zhang M, Pastore M, Li R, De Angelis F, Wang P (2012) Joint electrical, photophysical and computational studies on D-π-A dye sensitized solar cells: the impacts of dithiophene rigidification. Chem Sci 3(4):976–983. https://doi.org/10.1039/C2SC00973K
Chen S, Pei J, Pang Z, Wu W, Yu X, Zhang C (2020) Axial-symmetric conjugated group promoting intramolecular charge transfer performances of triphenylamine sensitizers for dye-sensitized solar cells. Dyes Pigments 174:108029. https://doi.org/10.1016/j.dyepig.2019.108029
Guo FL, Li ZQ, Liu XP, Zhou L, Kong FT, Chen WC, Dai SY (2016) Metal-free sensitizers containing hydantoin acceptor as high performance anchoring group for dye-sensitized solar cells. Adv Funct Mater 26(31):5733–5740. https://doi.org/10.1002/adfm.201601305
Chen M, Wang G-C, Yang W-Q, Yuan Z-Y, Qian X, Xu J-Q, Huang Z-Y, Ding A-X (2019) Enhanced synergetic catalytic effect of Mo2C/NCNTs@ Co heterostructures in dye-sensitized solar cells: fine-tuned energy level alignment and efficient charge transfer behavior. ACS Appl Mater Interfaces 11(45):42156–42171. https://doi.org/10.1021/acsami.9b14316
Rangan S, Katalinic S, Thorpe R, Bartynski RA, Rochford J, Galoppini E (2010) Energy level alignment of a zinc (II) tetraphenylporphyrin dye adsorbed onto TiO2 (110) and ZnO (1120) surfaces. J Phys Chem C 114(2):1139–1147. https://doi.org/10.1021/jp909320f
Kesavan R, Attia F, Su R, Anees P, El-Shafei A, Adhikari AV (2019) Asymmetric dual anchoring sensitizers/cosensitizers for dye sensitized solar cell application: an insight into various fundamental processes inside the cell. J Phys Chem C 123(40):24383–24395. https://doi.org/10.1021/acs.jpcc.9b06525
Zhu H-C, Li C-F, Fu Z-H, Wei S-S, Zhu X-F, Zhang J (2018) Increasing the open-circuit voltage and adsorption stability of squaraine dye binding onto the TiO2 anatase (1 0 1) surface via heterocyclic anchoring groups used for DSSC. Appl Surf Sci 455:1095–1105. https://doi.org/10.1016/j.apsusc.2018.06.081
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminformatics 4(1):17. https://doi.org/10.1186/1758-2946-4-17
Becke AD (1996) Density-functional thermochemistry. IV A new dynamical correlation functional and implications for exact-exchange mixing. J Chem Phys 104(3):1040–1046. https://doi.org/10.1063/1.470829
Granovsky AA (n.d.) Firefly version 8, http://classic.chem.msu.su/gran/firefly/index.html
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S (1993) General atomic and molecular electronic structure system. J Comput Chem 14(11):1347–1363. https://doi.org/10.1002/jcc.540141112
Irfan A (2019) Comparison of mono-and di-substituted triphenylamine and carbazole based sensitizers@(TiO2)38 cluster for dye-sensitized solar cells applications. Comput Theor Chem 1159:1–6. https://doi.org/10.1016/j.comptc.2019.04.008
Quarti C, Villafiorita-Monteoleone F, Botta C, Daita V, Perdicchia D, Del Buttero P, Del Zoppo M (2014) A spectroscopic study of the optical properties of a nitrobenzoxadiazole derivative in solution: the role of specific interactions. Chem Phys Lett 610:357–362. https://doi.org/10.1016/j.cplett.2014.07.042
Neese F (2008) ORCA-an ab initio density functional, and semiempirical program package, version 2.7. University of Bonn
Neese F (2018) Software update: the ORCA program system, version 4.0. Wiley Interdiscip Rev Comput Mol Sci 8(1):e1327. https://doi.org/10.1002/wcms.1327
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865. https://doi.org/10.1103/PhysRevLett.77.3865
Arkan F, Izadyar M (2018) Recent theoretical progress in the organic/metal-organic sensitizers as the free dyes, dye/TiO2 and dye/electrolyte systems; structural modifications and solvent effects on their performance. Renew Sust Energ Rev 94:609–655. https://doi.org/10.1016/j.rser.2018.06.054
Pastore M, De Angelis F (2013) Modeling materials and processes in dye-sensitized solar cells: understanding the mechanism, improving the efficiency. Multiscale modelling of organic and hybrid photovoltaics. Springer, pp 151–236. https://doi.org/10.1007/128_2013_468
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the damping function in dispersion corrected density functional theory. J Comput Chem 32(7):1456–1465. https://doi.org/10.1002/jcc.21759
Deskins NA, Dupuis M (2007) Electron transport via polaron hopping in bulk Ti O 2: a density functional theory characterization. Phys Rev B 75(19):195212
Roy JK, Kar S, Leszczynski J (2018) Insight into the optoelectronic properties of designed solar cells efficient tetrahydroquinoline dye-sensitizers on TiO 2 (101) surface: first principles approach. Sci Rep 8(1):1–12
Jedidi A, Markovits A, Minot C, Bouzriba S, Abderraba M (2010) Modeling localized photoinduced electrons in rutile-TiO2 using periodic DFT+ U methodology. Langmuir 26(21):16232–16238
Arroyo-de Dompablo M, Morales-García A, Taravillo M (2011) DFT+ U calculations of crystal lattice, electronic structure, and phase stability under pressure of TiO2 polymorphs. J Chem Phys 135(5):054503
Saranya G, Yam C, Gao S, Chen M (2018) Roles of chenodeoxycholic acid coadsorbent in anthracene-based dye-sensitized solar cells: a density functional theory study. J Phys Chem C 122(41):23280–23287
Liu Q-L, Zhao Z-Y, Liu Q-J (2014) Analysis of sulfur modification mechanism for anatase and rutile TiO2 by different doping modes based on GGA+ U calculations. RSC Adv 4(61):32100–32107. https://doi.org/10.1039/C4RA03891F
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13(12):5188. https://doi.org/10.1103/PhysRevB.13.5188
Kresse G, Furthmüller J (1996) Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci 6(1):15–50. https://doi.org/10.1016/0927-0256(96)00008-0
Kresse G, Hafner J (1994) Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys Rev B 49:14251. https://doi.org/10.1103/PhysRevB.49.14251
Liu H, Liu L, Fu Y, Liu E, Xue B (2019) Theoretical Design of D− π–A–A sensitizers with narrow band gap and broad spectral response based on boron dipyrromethene for dye-sensitized solar cells. J Chem Inf Model 59(5):2248–2256. https://doi.org/10.1021/acs.jcim.9b00187
Li P, Cui Y, Song C, Zhang H (2017) A systematic study of phenoxazine-based organic sensitizers for solar cells. Dyes Pigments 137:12–23. https://doi.org/10.1016/j.dyepig.2016.09.060
J-i F, Eda T, Hanaya M (2017) Comparative study of conduction-band and valence-band edges of TiO2, SrTiO3, and BaTiO3 by ionization potential measurements. Chem Phys Lett 685:23–26. https://doi.org/10.1016/j.cplett.2017.07.031
Preat J, Hagfeldt A, Perpète EA (2011) Investigation of the photoinduced electron injection processes for p-type triphenylamine-sensitized solar cells. Energy Environ Sci 4(11):4537–4549. https://doi.org/10.1039/C1EE01638E
Zanjanchi F, Beheshtian J (2019) Natural pigments in dye-sensitized solar cell (DSSC): a DFT-TDDFT study. J Iran Chem Soc 16(4):795–805. https://doi.org/10.1007/s13738-018-1561-2
Wei S, Li K, Lu X, Zhao Z, Shao Y, Dang Y, Li S, Guo W (2016) Theoretical insight into electronic structure and optoelectronic properties of heteroleptic Cu (I)-based complexes for dye-sensitized solar cells. Mater Chem Phys 173:139–145. https://doi.org/10.1016/j.matchemphys.2016.01.049
Tripathi A, Prabhakar C (2018) Impact of replacement of the central benzene ring in anthracene by a heterocyclic ring on electronic excitations and reorganization energies in anthratetrathiophene molecules. J Chin Chem Soc 65(8):918–924. https://doi.org/10.1002/jccs.201700448
Siddiqui SA (2019) In silico investigation of the coumarin-based organic semiconductors for the possible use in organic electronic devices. J Phys Org Chem 32(3):e3905. https://doi.org/10.1002/poc.3905
Tripathi A, Chetti P (2020) Enhanced charge transport properties in heteroatomic (NH, O, Se) analogs of benzotrithiophene (BTT) isomers: a DFT insight. Mol Simul 46(7):548–556. https://doi.org/10.1080/08927022.2020.1738425
Zhang M, Hua Z, Liu W, Liu H, He S, Zhu C, Zhu Y (2020) A DFT study on the photoelectric properties of rubrene and its derivatives. J Mol Model 26(2):32. https://doi.org/10.1007/s00894-020-4295-x
Fahim ZME, Bouzzine SM, Aicha YA, Bouachrine M, Hamidi M (2018) The bridged effect on the geometric, optoelectronic and charge transfer properties of the triphenylamine–bithiophene-based dyes: a DFT study. Res Chem Intermed 44(3):2009–2023. https://doi.org/10.1007/s11164-017-3211-1
He L-J, Wei W, Chen J, Jia R, Wang J, Zhang H-X (2017) The effect of D–[D e–π–A] n (n= 1, 2, 3) type dyes on the overall performance of DSSCs: a theoretical investigation. J Mater Chem C 5(30):7510–7520. https://doi.org/10.1039/C7TC02499A
Chermette H (1999) Chemical reactivity indexes in density functional theory. J Comput Chem 20(1):129–154. https://doi.org/10.1002/(SICI)1096-987X(19990115)20:1<129::AID-JCC13>3.0.CO;2-A
Gazquez JL, Cedillo A, Vela A (2007) Electrodonating and electroaccepting powers. J Phys Chem A 111(10):1966–1970. https://doi.org/10.1021/jp065459f
Moia D, Vaissier V, López-Duarte I, Torres T, Nazeeruddin MK, O'Regan BC, Nelson J, Barnes PR (2014) The reorganization energy of intermolecular hole hopping between dyes anchored to surfaces. Chem Sci 5(1):281–290. https://doi.org/10.1039/c3sc52359d
Vaissier V, Barnes P, Kirkpatrick J, Nelson J (2013) Influence of polar medium on the reorganization energy of charge transfer between dyes in a dye sensitized film. Phys Chem Chem Phys 15(13):4804–4814. https://doi.org/10.1039/C3CP44562C
Haque SA, Tachibana Y, Willis RL, Moser JE, Grätzel M, Klug DR, Durrant JR (2000) Parameters influencing charge recombination kinetics in dye-sensitized nanocrystalline titanium dioxide films. J Phys Chem B 104(3):538–547. https://doi.org/10.1021/jp991085x
Balanay MP, Kim DH (2009) Structures and excitation energies of Zn–tetraarylporphyrin analogues: a theoretical study. J Mol Struct THEOCHEM 910(1–3):20–26. https://doi.org/10.1016/j.theochem.2009.06.010
Lin BC, Cheng CP, You Z-Q, Hsu C-P (2005) Charge transport properties of tris (8-hydroxyquinolinato) aluminum (III): why it is an electron transporter. J Am Chem Soc 127(1):66–67. https://doi.org/10.1021/ja045087t
Al-Qurashi OS, Wazzan NA, Obot I (2020) Exploring the effect of mono-and di-fluorinated triphenylamine-based molecules as electron donors for dye-sensitised solar cells. Mol Simul 46(1):41–53. https://doi.org/10.1080/08927022.2019.1668561
Chen P, Yum JH, Angelis FD, Mosconi E, Fantacci S, Moon S-J, Baker RH, Ko J, Nazeeruddin MK, Grätzel M (2009) High open-circuit voltage solid-state dye-sensitized solar cells with organic dye. Nano Lett 9(6):2487–2492. https://doi.org/10.1021/nl901246g
Pastore M, De Angelis F (2010) Aggregation of organic dyes on TiO2 in dye-sensitized solar cells models: an ab initio investigation. ACS Nano 4(1):556–562. https://doi.org/10.1021/nn901518s
Ambrosio F, Martsinovich N, Troisi A (2012) What is the best anchoring group for a dye in a dye-sensitized solar cell? J Phys Chem Lett 3(11):1531–1535. https://doi.org/10.1021/jz300520p
Acknowledgements
O. Al-Qurashi and N. Wazzan acknowledge King Abdulaziz University’s High-Performance Computing Centre (Aziz Supercomputer) (http://hpc.kau.edu.sa) for supporting the computation for the work described in this paper.
Availability of data and material
Materials will be available on request.
Funding
G. Deogratias received financial support from the African Development Bank (AfDB), United Republic of Tanzania through project number P-Z1-IA0-016 and grant number 2100155032816.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deogratias, G., Al-Qurashi, O.S., Wazzan, N. et al. Investigation of optoelectronic properties of triphenylamine-based dyes featuring heterocyclic anchoring groups for DSSCs’ applications: a theoretical study. Struct Chem 31, 2451–2461 (2020). https://doi.org/10.1007/s11224-020-01596-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-020-01596-8