Abstract
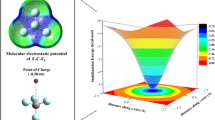
Detailed electrostatic potential (ESP) analyses were performed to compare the directionality of halogen bonds with those of hydrogen bonds and lithium bonds. To do this, the interactions of HOOOH with the molecules XF (X = Cl, Br, H, Li) were investigated. For each molecule, the percentage of the van der Waals (vdW) molecular surface that intersected with the ESP surface was used to roughly quantify the directionality of the halogen/hydrogen/lithium bond associated with the molecule. The size of the region of intersection was found to increase in the following order: ClF < BrF < HF < LiF. The maximum ESP in the region of intersection, V S, max, was observed to become more positive according to the sequence ClF < BrF < HF < LiF. For ClF and BrF, the positive electrostatic potential was concentrated in a very small region of the vdW molecular surface. On the other hand, for HF and LiF, the positive electrostatic potential was more diffusely scattered across the vdW surface than for ClF and BrF. Also, the optimized geometries of the dipolymers HOOOH··· XF (X = Cl, Br, H, Li) indicated that halogen bonds are more directional than hydrogen bonds and lithium bonds, consistent with the results of ESP analyses.

Electrostatic potential (ESP) contour maps in the xz plane of ClF and BrF





Similar content being viewed by others
References
Politzer P, Murray JS, Clark T (2013) Phys Chem Chem Phys 15:11178–11189
Politzer P, Murray JS (2013) ChemPhysChem 14:278–294
Pierangelo M, Giuseppe R (2015) Halogen bonding II: impact on materials chemistry and life sciences. Topics in current chemistry, vol 359. Springer, Cham
Gilday LC, Robinson SW, Barendt TA, Langton MJ, Mullaney BR, Beer PD (2015) Chem Rev 115:7118–7195
Li QZ, Li HB (2015) Noncovalent forces. Springer, Switzerland
Ji J, Zeng Y, Zhang X, Zheng S, Meng L (2013) J Mol Model 19:4887–4895
Pauling L (1960) The nature of the chemical bond, 3rd edn. Cornell University Press, Ithaca
Allen LC (1989) J Am Chem Soc 111:9003–9014
Murray-Rust P, Stallings WC, Monti CT, Preston RK, Glusker JP (1983) J Am Chem Soc 105:3206–3214
Clark T, Hennemann M, Murray JS, Politzer P (2007) J Mol Model 13:291–296
Shields ZP, Murray JS, Politzer P (2010) Int J Quantum Chem 110:2823–2832
Zeng Y, Zhu M, Meng L, Zheng S (2011) ChemPhysChem 12:3584–3590
Zeng Y, Zhang X, Li X, Meng L, Zheng S (2011) ChemPhysChem 12:1080–1087
Zeng Y, Li X, Zhang X, Zheng S, Meng L (2011) J Mol Model 17:2907–2918
Li X, Zeng Y, Zhang X, Zheng S, Meng L (2011) J Mol Model 17:757–767
Cerkovnik J, Plesnicar B (2013) Chem Rev 113:7930–7951
Hagelin H, Murray JS, Politzer P, Brinck T, Berthelot M (1995) Can J Chem 73:483–488
Murray JS, Politzer P (1998) J Mol Struct THEOCHEM 425:107–114
Politzer P, Murray JS (2001) Fluid Phase Equilib 185:129–137
Stewart RF (1979) Chem Phys Lett 65:335–342
Politzer P, Truhlar DG (1981) Chemical applications of atomic and molecular electrostatic potentials. Plenum, New York
Scrocco E, Tomasi J (1973) The electrostatic molecular potential as a tool for the interpretation of molecular properties. Topics in current chemistry. Springer, Berlin
Lu T, Chen F (2012) J Comput Chem 33:580–592
Politzer P, Lane P, Concha MC, Ma Y, Murray JS (2007) J Mol Model 13:305–311
Murray JS, Politzer P (1987) Theor Chim Acta 72:507–517
Politzer P, Laurence PR, Jayasuriya K (1985) Environ Health Perspect 61:191–202
Naray-Szabo G, Ferenczy GG (1995) Chem Rev 95:829–847
Boys SF, Bernardi F (1970) Mol Phys 19:553–566
Frisch MJ, Trucks GW, Schlegel HB et al (2009) Gaussian 09, revision A.02. Gaussian Inc., Wallingford
Lu T, Chen F (2013) J Mol Model 19:5387–5395
Bulat FA, Toro-Labbe A, Brinck T, Murray JS, Politzer P (2010) J Mol Model 16:1679–1691
Lu T, Chen F (2012) J Mol Graph Model 38:314–323
Bader RFW, Larouche A, Gatti C, Carroll MT, MacDougall PJ, Wiberg KB (1987) J Chem Phys 87:1142
Alvarez S (2013) Dalton Trans 42:8617–8636
Acknowledgments
Contract grant sponsors: National Natural Science Foundation of China (21371045 and 21373075), Natural Science Foundation of Hebei Province (B2015205045 and B2014205109), Education Department Foundation of Hebei Province (ZH2012106 and ZD20131037).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors have full access to all of the raw data used in this work, and the authors agree to allow the journal to review their data if requested.
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Liu, L., Meng, L., Zhang, X. et al. Comparison of the directionality of the halogen, hydrogen, and lithium bonds between HOOOH and XF (X = Cl, Br, H, Li). J Mol Model 22, 52 (2016). https://doi.org/10.1007/s00894-016-2919-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-2919-y