Abstract
Thirteen X-ray crystal structures containing various non-covalent interactions such as halogen bonds, halogen–halogen contacts and hydrogen bonds (I⋯N, I⋯F, I⋯I, F⋯F, I⋯H and F⋯H) were considered and investigated using the DFT-D3 method (B97D/def2-QZVP). The interaction energies were calculated at MO62X/def2-QZVP and MP2/aug-cc-pvDZ level of theories. The higher interaction and dispersion energies (2nd crystal) of −9.58 kcal mol−1 and −7.10 kcal mol−1 observed for 1,4-di-iodotetrafluorobenzene bis [bis (2-phenylethyl) sulfoxide] structure indicates the most stable geometrical arrangement in the crystal packing. The electrostatic potential values calculated for all crystal structures have a positive σ-hole, which aids understanding of the nature of σ-hole bonds. The significance of the existence of halogen bonds in crystal packing environments was authenticated by replacing iodine atoms by bromine and chlorine atoms. Nucleus independent chemical shift analysis reported on the resonance contribution to the interaction energies of halogen bonds and halogen–halogen contacts. Hirshfeld surface analysis and topological analysis (atoms in molecules) were carried out to analyze the occurrence and strength of all non-covalent interactions. These analyses revealed that halogen bond interactions were more dominant than hydrogen bonding interactions in these crystal structures.

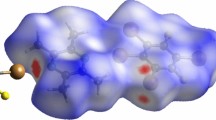
Molecluar structure of 1,4-Di-iodotetrafluorobenzene bis(thianthrene 5-oxide) moelcule and its corresponding molecular electrostatic potential map for the view of σ-hole.

















Similar content being viewed by others
References
Auffinger P, Hays FA, Westhof E, Ho PS (2004) Proc Natl Acad Sci USA 101:16789
Voth AR, Hays FA, Ho PS (2007) Proc Natl Acad Sci USA 104:6188
Jentzsch AV, Emery D, Mareda J, Nayak SK, Metrangolo P, Resnati G, Sakai N, Matile S (2012) Nat Commun 3:905
Caballero A, Zapata F, White NG, Costa PJ, Faclix VT, Beer PD (2012) Angew Chem 124:1912
El-Sheshtawy HS, Bassil BS, Assaf KI, Kortz U, Nau WM (2012) J Am Chem Soc 134:19935–19941
Lu Y, Shi T, Wang Y, Yang H, Yan X, Luo X, Jiang H, Zhu W (2009) J Med Chem 52:2854
Zha B, Miao X, Liu P, Wu Y, Deng W (2014) Chem Commun 50:9003
Nguyen HL, Horton PN, Hursthouse MB, Legon AC, Bruce DW (2004) J Am Chem Soc 126:16
Meazza L, Foster JA, Fucke K, Metrangolo P, Resnati G, Steed JW (2013) Nat Chem 5:42
Kawai S, Sadeghi A, Xu F, Peng L, Orita A, Otera J, Goedecker S, Meyer E (2015) ACS Nano 9:2574
Price S, Stone A, Lucas J, Rowland R, Thornley A (1994) J Am Chem Soc 116:4910
McDowell SA, Joseph JA (2015) Mol Phys 113:16
Zhou P-P, Qiu W-Y, Liu S, Jin N-Z (2011) Phys Chem Chem Phys 13:7408
Murray-Rust P, Motherwell WS (1979) J Am Chem Soc 101:4374
Deepa P, Pandiyan BV, Kolandaivel P, Hobza P (2014) Phys Chem Chem Phys 16:2038
Deepa P, Sedlak R, Hobza P (2014) Phys Chem Chem Phys 16:6679
Sedlak R, Deepa P, Hobza P (2014) J Phys Chem A 118:3846
Pandiyan BV, Deepa P, Kolandaivel P (2014) Phys Chem Chem Phys 16:19928
Pandiyan BV, Deepa P, Kolandaivel P (2014) Phys Chem Chem Phys 17:27496
Kolr MH, Deepa P, Ajani H, Pecina A, Hobza P (2015) Top Curr Chem 359:1
Meyer F, Dubois P (2013) CrystEngComm 15:3058
Andrews MB, Cahill CL (2012) Dalton Trans 41:3911
Paton AS, Lough AJ, Bender TP (2011) CrystEngComm 13:3653
Wallnoefer HG, Fox T, Liedl KR, Tautermann CS (2010) Phys Chem Chem Phys 12:14941
Pigge FC, Vangala VR, Kapadia PP, Swenson DC, Rath NP (2008) Chem Commun 39:4726
Jetti RK, Nangia A, Xue F, Mak TC (2001) Chem Commun 2001:919
Riley KE, Murray JS, Fanfrlik JI, Rezac J, Sola RJ, Concha MC, Ramos FM, Politzer P (2013) J Mol Model 17:3309
Clark T, Hennemann M, Murray JS, Politzer P (2007) J Mol Model 13:291
Politzer P, Murray JS, Clark T (2010) Phys Chem Chem Phys 12:7748
Politzer P, Murray JS, Clark T (2013) Phys Chem Chem Phys 15:11178
McDowell SA, Joseph Physical JA (2014) Chem Chem Phys 16:10854
Riley KE, Merz KM (2007) J Phys Chem A 111:1688
Metrangolo P, Neukirch H, Pilati T, Resnati G (2005) Acc Chem Res 38:386
Grimme S, Ehrlich S, Goerigk L (1997) J Comput Chem 32:1456
Gaussian 09, Revision D.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian, Inc., Wallingford CT
Chambers J, Migliorini F (1997) Bull Am Astronom Soc 29:1024
Murray JS, Lane P, Politzer P (2009) J Mol Model 15:723
Bader RF, Carroll MT, Cheeseman JR, Chang C (1987) J Am Chem Soc 109:7968
Zahedi E, Pangh A, Ghorbanpour H (2015) Surf Rev Lett 22:1550005
Steiner T (2002) Angew Chem Int Ed 41:48
Hirshfeld FL (1977) Theor Chim Acta 44:12
Spackman MA, Jayatilaka D (2009) CrystEngComm 11:19
Spackman MA, McKinnon JJ (2002) CrystEngComm 4:378
Wang R, Dols TS, Lehmann CW, Englert U (2012) Chem Commun 48:6830
McKinnon JJ, Jayatilaka D, Spackman MA (2007) Chem Commun 196:3814
Roohi H, Anjomshoa E (2011) Bull Chem Soc Jpn 84:754
Gilday LC, Robinson SW, Barendt TA, Langton MJ, Mullaney BR, Beer PD (2015) Chem Rev 115:7118
Pandiyan BV, Deepa P, Kolandaivel P (2016) Mol Phys. doi: 10.1080/00268976.2016.1255796
Vijaya Pandiyan B, Kolandaivel P, Deepa P (2014) Mol Phys 112:1609
Acknowledgment
This work was part of the Research Project (File Number: YSS/2015/000275) and P.D. is thankful to the Science and Engineering Research Board (SERB), Government of India, New Delhi for the award of this Project.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Pandiyan, B.V., Deepa, P. & Kolandaivel, P. How do halogen bonds (S–O⋯I, N–O⋯I and C–O⋯I) and halogen–halogen contacts (C–I⋯I–C, C–F⋯F–C) subsist in crystal structures? A quantum chemical insight. J Mol Model 23, 16 (2017). https://doi.org/10.1007/s00894-016-3181-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-3181-z