Abstract
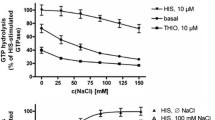
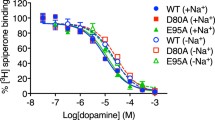
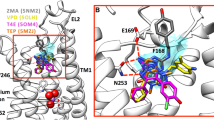
Several aminergic GPCRs, e.g., the human histamine H3-receptor (hH3R) are sensitive to sodium ions. Based on these experimental results, including site directed mutagenesis studies, a sodium binding pocket near to the highly conserved Asp2.50 was suggested. Recently, in the crystallized adenosine A2A receptor (4EIY), a sodium ion was found in a pocket, coordinated by Asp52, Ser91, and three water molecules. Despite high homology in amino acid sequence between hH3R and hH4R, pharmacological studies revealed that the hH4R is — in contrast to hH3R — not sensitive to sodium ions. In order to obtain a deeper insight onto the differences in sodium sensitivity between hH3R and hH4R, we performed molecular modelling studies, including molecular dynamic simulations and calculation of Gibbs energy of solvation. The results of the modeling studies suggested that the amino acid at position 7.42 influences sodium binding to aminergic GPCRs in different ways. A comparison of the amino acids forming the sodium binding channel between the ligand binding pocket and the sodium binding pocket of all human aminergic GPCRs showed an 80 % occurrence of glycine — in contrast to hH3R and hH4R. The Gln7.42 at hH4R disrupts a water chain, connecting the Asp3.32 of the orthosteric binding site and the Asp2.50 of the allosteric binding site. Besides, the oxygen of the glutamine side chain stabilizes the interaction of the sodium ion with the Asp3.32. Thus, the binding of the sodium into the allosteric binding site might be hindered kinetically.

Binding of Na+ to hH3R






Similar content being viewed by others
References
Foord SM, Bonner TI, Neubig RR, Rosser EM, Pin JP, Davenport AP, Spedding M, Harmar AJ (2005) International Union of Pharmacology. XLVI. G protein-coupled receptor list. Pharmacol Rev 57:279–288
Wise A, Gearing K, Rees S (2002) Target validation of G-protein coupled receptors. Drug Discov Today 7:235–246
Jacoby E, Bouhelal R, Gerspacher M, Seuwen K (2006) The 7TM G-protein-coupled receptor target family. Chem Med Chem 1:760–782
Hill SJ, Ganellin CR, Timmermann H, Schwartz JC, Shankley NP, Young JM et al. (1997) International Union of Pharmacology XIII. Classification of histamine receptors. Pharmacol Rev 49:253–278
Thurmond RL, Gelfand EW, Dunford PPJ (2008) The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat Rev Drug Discov 7:41–53
Tiligada E, Zampeli E, Sander K, Stark H (2009) Histamine H3 and H4 receptors as novel drug targets. Expert Opin Investig Drugs 18:1519–1531
Leurs R, Vischer HF, Wijtmans M, de Esch IJ (2011) En route to new blockbuster anti-histamines: surveying the offspring of the expanding histamine receptor family. Trends Pharmacol Sci 32:250–257
Strasser A, Wittmann HJ, Buschauer A, Schneider EH, Seifert R (2013) Species-dependent activities of G-protein-coupled receptor ligands: lessons from histamine receptor orthologs. Trends Pharmacol Sci 34:13–32
Seifert R, Strasser A, Schneider EH, Neumann D, Dove S, Buschauer A (2013) Moleclar and cellular analysis of human histamine receptor subtypes. Trends Pharmacol Sci 32:33–58
Lefkowitz RJ, Cotecchia S, Samama P, Costa T (1993) Constitutive activity of receptors coupled to guanine nucleotide regulatory proteins. Trends Pharmacol Sci 14:303–307
Leff P (1995) The two-state model of receptor activation. Trends Pharmacol Sci 16:89–97
Schütz W, Freissmuth M (1992) Reverse intrinsic activity of antagonists on G protein-coupled receptors. Trends Pharmacol Sci 13:376–380
Seifert R, Wenzel-Seifert K (2002) Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild type receptors. Naunyn Schmiedeberg's Arch Pharmacol 366:381–416
Seifert R, Wenzel-Seifert K (2003) The human formyl peptide receptor as model system for constitutively active G-protein-coupled receptors. Life Sci 73:2263–2280
Seifert R, Wenzel-Seifert K (2001) Unmasking different constitutive activity of four chemoattractant receptors using Na+ as universal stabilizer of the inactive (R) state. Receptor Channels 7:357–369
Martin S, Botto JM, Vincent JP, Mazella J (1999) Pivotal role of an aspartate residue in sodium sensitivity and coupling to G proteins of neurotensin receptors. Mol Pharmacol 55:210–215
Ericksen SS, Cummings DF, Weinstein H, Schetz JA (2009) Ligand selectivity of D2 dopamine receptors is modulated by changes in local dynamics produced by sodium binding. J Pharmacol Exp Ther 328:40–54
Schetz JA (2005) Allosteric modulation of dopamine receptors. Mini Rev Med Chem 5:555–561
Neve K (1991) Regulation of dopamine D2 receptors by sodium and pH. Mol Pharmacol 39:570–578
Schnell D, Seifert R (2010) Modulation of histamine H3 receptor function by monovalent ions. Neuroscience Lett 472:114–118
Schneider EH, Seifert R (2009) Histamine H4 receptor-RGS fusion proteins expressed in Sf9 insect cells: a sensitive and reliable approach for the functional characterization of histamine H4 receptor ligands. Biochem Pharmacol 78:607–616
Neve KA, Cox BA, Henningsen RA, Spanoyannis A, Neve RL (1991) Pivotal role for aspartate-80 in the regulation of dopamine D2 receptor affinity for drugs and inhibition of adenylyl cyclase. Mol Pharmacol 39:733–739
Schetz JA, Sibley DR (2001) The binding-site crevice of the D4 dopamine receptor is coupled to three distinct sites of allosteric modulation. J Pharmacol Exp Ther 296:359–363
Ceresa BP, Limbird LE (1994) Mutation of an aspartate residue highly conserved among G-protein-coupled receptors results in nonreciprocal disruption of α2-adrenergic receptor-G-protein interactions. J Biol Chem 269:29557–29564
Liu W, Chun E, Thompson AA, Chubukov P, Xu F, Katritch V, Han GW, Roth CB, Heitman LH, Ijzerman AP, Cherezov V, Stevens RC (2012) Structural basis for allosteric regulation of GPCRs by sodium ions. Science 337:232–235
Selent J, Sanz F, Pastor M, De Fabritiis G (2010) Induced effects of sodium ions on dopaminergic G-protein coupled receptors. Plos Comput Chem 6:e10000884
Yuan S, Vogel H, Filipek S (2013) The role of water and sodium ions in the activation of the μ-opioid receptor. Angew Chem Int Ed 52:10112–10115
Shimamura T, Shiroishi M, Weyand S, Tsujimoto H, Graeme W, Katritch V, Abagyan R, Cherezov V, Liu W, Han GW, Kobayahi T, Stevens RS, Iwata S (2011) Structure of the human histamine H1 receptor complex with doxepin. Nature 475:65–70
Strasser A, Wittmann HJ (2013) Molecular modeling studies give hint for the existence of a symmetric hβ2R-Gαβγ-homodimer. J Mol Model 19:4443–4457
Angel TE, Chance MR, Palczewski K (2009) Conserved waters mediate structural and functional activation of family A (rhodopsin-like) G protein-coupled receptors. PNAS 106:8555–8560
Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC (2007) High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science 318:1258–1265
Wagner E, Wittmann HJ, Elz S, Strasser A (2013) Pharmacologcial profile of astemizole derived compounds at the histamine H1 and H4 receptor – H1/H4-receptor selectivity. Naunyn Schmiedeberg's Arch Pharmacol. doi:10.1007/s00210-013-0926-4
Humphrey W, Dalke A, Schulten K (1996) VMD – visual molecular dynamics. J Mol Graphics 14:33–38
Oostenbrink C, Villa A, Mark AE, van Gunsteren WF (2004) A biomolecular force field based on the free enthalpy of hydration and solvation: the GROMOS force-field parameter sets 53A5 and 53A6. J Comput Chem 25:1656–1676
Straatsma TP, Mc Cammon JA (1991) Multiconfiguration thermodynamic integration. J Chem Phys 95:1175–1188
Villa A, Mark AE (2002) Calculation of the free energy of solvation for natural analogs of amino acid side chains. J Comput Chem 23:548–553
Wittmann HJ, Elz S, Seifert R, Strasser A (2011) Nα-Methylated phenylhistamines exhibit affinity to the hH4R – a pharmacological and molecular modelling study. Naunyn-Schmiedeberg’s Arch Pharmacol 384:287–299
Wittmann HJ, Seifert R, Strasser A (2014) Mathematical analysis of the sodium sensitivity of the human histamine H3 receptor. In silico pharmacology, accepted
Li W, Zhang J, Wang J, Wang W (2008) Metal-coupled folding of Cys2His2 Zinc-finger. J Am Chem Soc 130:892–900
Strasser A, Wittmann HJ, Seifert R (2008) Ligand-specific contribution of the N-terminus and E2-loop to pharmacological properties of the histamine H1-receptor. J Pharmacol Exp Ther 326:783–791
Yao BB, Hutchins CW, Carr TL, Cassar S, Masters JN, Bennani YL et al (2003) Molecular modelling and pharmacological analysis of species-related histamine H3 receptor heterogeneity. Neuropharmacology 44:773–786
Acknowledgments
This work was supported by DFG (STR 1125/1-1) of the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Alignment of the amino acid sequences of the hA2AR, hβ2R, hH3R, and hH4R. Gray shaded: highly conserved amino acid within each TM domain; yellow shaded: highly conserved cysteine, forming a disulfide bond between the E2-loop and the upper part of TM III; red: amino acids, different between hH3R and hH4R and suggested to be responsible for the observed subtype differences in sodium sensitivity, based on molecular modeling studies. (GIF 75 kb)
Fig. S2
Relative density profile of water and the lipid bilayer. The relative density profile of water and the nitrogens of the POPC lipid bilayer is given as mean value with respect to time for the productive simulation phase of the hH3R. The relative density is calculated independently for the water and the POPC-nitrogen in such a manner that the maximum for both curves is 1. The gray line behind the curve for water indicates the relative water density at 100 different time frames within the productive simulation. (GIF 222 kb)
Fig. S3
Schematic presentation of the lattice used for calculation of the Coulomb potential and Coulomb- and Lennard-Jones interaction surface. Every 10th lattice point is shown as a black dot. Only one exemplary lattice is shown along the y-axis. (GIF 197 kb)
Fig. S4
Hydration numbers for the sodium ion during the binding to hH3R and hH4R. The mean number of oxygens (from water or protein) within a distance less than 0.25 nm, calculated according to “Materials and methods” for distinct penetration states of the sodium ion to hH3R or hH4R. (GIF 76 kb)
Fig. S5
Snapshots of MD simulations regarding the hydration of the sodium ion. The change of the hydration environment of the Na+ is shown exemplary for the orthosteric binding site near Asp3.32 of hH4R. a, the sodium ion is coordinated by the two carboxylic oxygens of Asp3.32 and three water molecules. b, schematic presentation of the rotation of a Na+-H2O unit during the further binding process. c, the sodium ion is hydrated by five water molecules. (GIF 147 kb)
Fig. S6
Alignment of the amino acids, forming the sodium binding channel of the human A2A receptor, mouse μ-opioid receptor and all human aminergic GPCRs. All amino acids, forming the sodium binding channel, are presented within green or yellow boxes. Green: the most conserved amino acid at that position; yellow: amino acids, which are different to the most conserved amino acid at that position. (GIF 242 kb)
Fig. S7
Direct view from Asp3.32 into the sodium channel based on molecular dynamic simulations of hH3R, hH4R-Gln7.42Leu, hH4R-Gln7.42Gly, and hH4R. Shown are snapshots by molecular dynamic simulations. (GIF 192 kb)
Fig. S8
Influence of Val3.40 in hH4R and Ala3.40 in an hH4R-Val3.40Ala mutant onto the sodium binding pocket. Shown are snapshots obtained by molecular dynamic simulations. (GIF 97 kb)
Fig. S9
Influence of the conformation of the Gln7.42 side chain in hH4R onto electrostatic potential in hH4R. a, There is no sodium in the allosteric binding site. b, A sodium ion is present in the allosteric binding site. Shown are snapshots obtained by molecular dynamic simulation. A negative potential is indicated by blue, a more positive potential is indicated by red. (GIF 71 kb)
Rights and permissions
About this article
Cite this article
Wittmann, HJ., Seifert, R. & Strasser, A. Sodium binding to hH3R and hH4R — a molecular modeling study. J Mol Model 20, 2394 (2014). https://doi.org/10.1007/s00894-014-2394-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2394-2