Abstract
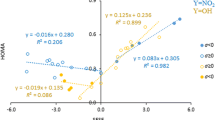
Interactions between the NO2 group and 13 different substituents (BF2, BH2, CF3, CH3, CHO, CN, F, NH2, NMe2, NO2, NO, OH, OMe) were investigated computationally for bicyclo[2.2.2]octane (BCO) and benzene substituted at 1,4 and 1,3 positions in the ring. Three methods were employed to estimate the character and strength of the substituent effect: substituent effect stabilization energy (SESE), sigma/pi electron donor acceptor index (sEDA/pEDA) and substituent active region (cSAR) parameter. For the first time the sEDA/pEDA parameters were calculated not for the ring but for the NO2 group. All calculations were performed at the B3LYP/6-31G(d,p) level of theory. For 1,4 derivatives, a direct comparison of slopes of linear regressions between BCO and benzene reveals a much better transmission of the substituent effect in the latter. The ratio of slopes (benzene over BCO) is always larger than 4. It follows that the resonance effects, which are absent in the BCO, dominate in this case. For 1,3 derivatives, because of much lower correlation coefficients, estimated standard deviations (ESD) were used to calculate the ratio instead of the slopes. For these systems the ratio is much closer to the unity, which indicates that only the sigma/through space effects are present and they are of similar magnitude in benzene and BCO. It follows from natural population analysis (NPA) charges that the substituent effect in the studied systems is due mainly to through-space interactions.

What is the nature of the substituent effect











Similar content being viewed by others
Notes
Explanation: In our two recent publications the symbol qSAR was used for charge of Substituent Active Region. However, to avoid confusion with QSAR meaning Quantitative Structure Activity Relationships we decided to used the symbol cSAR instead.
References
Hammett LP (1937) J Am Chem Soc 59:96
Hammett LP (1935) Chem Rev 17:125
Hammett LP (1940) Physical organic chemistry, 1st edn. McGraw-Hill, New York, pp 194–228
Jaffe HH (1953) Chem Rev 53:191
Palm VA (1967) Osnovy kolichestvennnoy teoryi organitcheskikh soedinenii, Izd. Leningrad, Khimya
Exner O (1972) The Hammett equation—the present position. In: Chapman NB, Shorter J (eds) Advances in linear free energy relationships. Plenum, London, pp 1–69
Johnson CD (1973) The Hammett equation. University Press, Cambridge
Charton M (1973) Progr Phys Org Chem 10:81
Shorter J (1991) Substituent effect parameters and models applied inorganic chemistry. In: Zalewski RI, Krygowski TM, Shorter J (eds) Similarity models in organic chemistry, biochemistry and related fields. Elsevier, Amsterdam, pp 77–148
Hansch C, Leo A, Taft RW (1991) Chem Rev 91:165
Krygowski TM, Stępień BT (2005) Chem Rev 105:3482
Exner O, Bohm S (2006) Curr Org Chem 10:763
Exner O (1978) Chapter 10. In: Chapman NB, Shorter J (eds) Correlation analysis in chemistry. Plenum, London
Ehrenson S, Brownlee RTC, Taft RW (1973) Progr Phys Org Chem 10:1
Brown HC, Okamoto Y (1958) J Am Chem Soc 90:4979
Wells PR (1968) Linear free energy relationships. Academic, London
Zuman P (1967) Substituent effects in organic polarography. Plenum, New York
Williams A (2003) Free energy relationships in organic and bio-organic chemistry. Royal Society of Chemistry, Cambridge
Ingold CK (1969) Structure and mechanism in organic chemistry, 2nd edn. Cornell University Press, Ithaca
Roberts JD, Moreland WT (1953) J Am Chem Soc 75:2167
Palecek J, Hlavaty J (1973) Coll Czech Chem Comm 38:1985
Grob CA, Schlegeter MG (1974) Helv Chim Acta 57:509
Taft RW, Lewis IC (1958) J Am Chem Soc 80:2436
Taft RW, Lewis IC (1959) J Am Chem Soc 81:5343
Taft WR, Price E, Fox IR, Lewis IC, Andersen KK, Davis GT (1963) J Am Chem Soc 85:709
Holtz HD, Stock LM (1964) J Am Chem Soc 96:4555
Taft RW, Topsom RD (1987) Progr Phys Org Chem 16:1
Charton M (1984) J Org Chem 49:1997
Taylor PJ, Wait AR (1986) J Chem Soc Perkin 2:1765
Adcock W, Anvia F, Butt G, Cook A, Duggan P, Grob CA, Marriott S, Rowe J, Taagapera M, Taft RW, Topsom RW (1991) J Phys Org Chem 4:353
Wiberg KB (2002) J Org Chem 67:4787
Alkorta I, Griffiths MZ, Popelier PLA (2013) J Phys Org Chem 26:791
Exner O, Krygowski TM (1996) Chem Soc Rev 71.
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899
Oziminski WP, Dobrowolski JC (2009) J Phys Org Chem 22:769
Sadlej-Sosnowska N (2007) Pol J Chem 81:1123
Sadlej-Sosnowska N (2007) Chem Phys Lett 447:192
Krygowski TM, Sadlej-Sosnowska N (2011) Struct Chem 22:17
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision C.02. Gaussian Inc, Wallingford
Pross A, Radom L, Taft RW (1980) J Org Chem 45:818
Hehre WJ, Radom L, Schleyer PR, Pople AJ (1986) Ab initio molecular orbital theory. Wiley, New York, p 360
Snedecor GW, Cochran WG (1973) Statistical methods. Iowa State University Press, Ames
Acknowledgments
A computational grant from the Wroclaw Centre for Networking and Supercomputing (WCSS) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 272 kb)
Rights and permissions
About this article
Cite this article
Krygowski, T.M., Oziminski, W.P. Substituent effects in 1-nitro-4-substituted bicyclo[2.2.2]octane derivatives: inductive or field effects?. J Mol Model 20, 2352 (2014). https://doi.org/10.1007/s00894-014-2352-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2352-z