Abstract
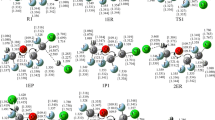
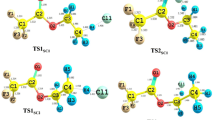
The mechanism and kinetics of 2,2,3,3,3-pentafluoropropanol (CF3CF2CH2OH) reaction with Chlorine atom (Cl) is investigated in this work. Two hydrogen abstraction channels of the title reaction are identified. The geometries of all the stationary points in the potential energy surface are obtained at the BHandHLYP/6-311G** level, and the energies of the selected points along the minimum energy path (MEP) are improved by the CCSD(T) method. A dual-level direct dynamics method is employed to study the kinetic nature of the hydrogen-abstraction reaction channels. The calculated rate coefficients show that the hydrogen abstraction from the CH2 group is the primary channel. The calculated total rate coefficients are in best agreement with the experimental values. The four-parameter rate coefficients expression of the title reaction between the temperatures 200 K and 1000 K is provided.





Similar content being viewed by others
References
Ravishankara AR, Turnipseed AA, Jensen NR, Barone S, Mills M, Howard CJ, Solomon S (1994) Science 263:71–75
WMO/UNEP, 2010: Scientific Assessment of Ozone Depletion
Furon T (1990) Manyuaru S. Fluorocarbon Manufacturers Association, Tokyo, Japan
Singh HB, Kasting JF (1988) J Atmos Chem 7:261–285
Spicer W, Chapman EG, Finlayson-Pitts BJ, Plastridge RA, Hubbe JM, Fast JD, Berkowitz CM (1998) Nature 394:353–356
Finlayson-Pitts BJ, Pitts JN (2000) Chemistry of the upper and lower atmosphere. Academic, New York
Tanaka PL, Riemer DD, Chang SH, Yarwood G, McDonald-Buller EC, Apel EC, Orlando JJ, Silva PJ, Jimenez JL, Canagaratna MR, Neece JD, Mullins CD, Allen DT (2003) Atmos Environ 37:1393–1400
Tanaka PL, Oldfield S, Neece JD, Mullins CB, Allen DT (2000) Environ Sci Technol 34:4470–4473
Tokuhashi K, Nagai H, Takahashi A, Kaise M, Kondo S, Sekiya A, Takahashi M, Gotoh Y, Suga A (1999) J Phys Chem A 103:2664–2672
Chen L, Fukuda K, Takenaka N, Bandow H, Maeda Y (2000) Int J Chem Kinet 32:73–78
Hurley MD, Wallington TJ, Sulbaek MP, Andersen S, Ellis DA, Martin JW, Mabury SA (2004) J Phys Chem A 108:1973–1979
Antinolo M, Gonzalez S, Ballesteros B, Albaladejo J, Jimenez E (2012) J Phys Chem A 116:6041–6050
Wang Y, Liu J, Li Z, Wang L, Wu J, Sun C (2006) J Phys Chem A 110:5853–5859
Papadimitriou VC, Papanastasiou DK, Stefanopoulos VG, Zaras AM, Lazarou YG, Papagiannakopoulos P (2007) J Phys Chem A 111:11608–11617
Garzon A, Antinolo M, Moral M, Notario A, Jimenez E, Fernandez-Gomez M, Albaladejo J (2013) Mol Phys 111:753–763
Becke AD (1993) J Chem Phys 98:1372–1377
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Yang L, Liu JY, Li ZS (2008) J Phys Chem A 112:6364–6372
Yu AY, Zhang HX (2013) Mol Phys doi:10.1007/s00894-013-1960-3
Scuseria GE, Schaefer HF (1989) J Chem Phys 90:3700–3703
Pople JA, Gordon MH, Raghavachari K (1989) J Chem Phys 87:5968–5975
Frisch MJ, Trucks GW, Schlegel HB et al. (2009) Gaussian 09. Revision X. Gaussian Inc, Wallingford, CT
Truhlar DG (1995) In: Heidrich D (Ed.) The reaction path in chemistry: current approaches and perspectives; Kluwer, Dordrecht, the Netherlands, p 229
Fernandez-Ramos A, Miller JA, Klippenstein SJ, Truhlar DG (2006) Chem Rev 106:4518–4584
Hu WP, Truhlar DG (1995) J Am Chem Soc 117:10726–10734
Garrett BC, Truhlar DG (1979) J Chem Phys 70:1593–1598
Liu YP, Lynch GC, Truong TN, Liu DH, Truhlar DG, Garrett BC (1993) J Am Chem Soc 115:2408–2415
Steckler R, Hu WP, Liu YP, Lynch GC, Garrett BC, Isaacson AD, Melissas VS, Lu DP, Troung TN, Rai SN, Hancock GC, Lauderdate JG (1995) Comput Phys Commun 88:341–343
Truhlar DG (1991) J Comp Chem 12:266–270
Chuang YY, Truhlar DG (2000) J Chem Phys 112:1221–1228
Liu YP, Gonzàlez-Lafont A, Truhlar DG, Garrett BC (1993) J Am Chem Soc 115:7806–7817
Truhlar DG, Isaacson AD, Garrett BC (1985) In Theory of Chemical Reaction Dynamics; Baer, M., Ed.; 4: 65-137
Truhlar DG, Isaacson AD, Skodje RT, Garrett BC (1982) J Phys Chem 86:2252–2261
Chang YY, Corchado JC, Fast PL, Villa J, Hu WP, Liu YP, Lynch GC, Jackels CF, Nguyen KA, Gu MZ, Rossi I, Coitino EL, Claylon S, Melissas VS, Lynch BJ, Steckler R, Garrett BC, Isaacson AD, Truhlar DG (2007) POLYRATE version 9.6. University of Minnesota, Minneapolis
Scheiner S, Seybold PG (2009) Struc Chem 20:43–48
Zheng J, Truhlar DG (2012) Faraday Discuss 157:59–88
Acknowledgments
This work is supported by the Natural Science Foundation of China (Grant No.20973076 and 21173096) and Specialized Research Fund for the Doctoral Program of Higher Education (20110061110018). Thanks are due to the reviewers for many valuable comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, Ay., Zhang, Hx. Direct dynamics simulations of the hydrogen abstraction reaction Cl + CF3CF2CH2OH. J Mol Model 19, 4503–4510 (2013). https://doi.org/10.1007/s00894-013-1960-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-013-1960-3