Abstract
Phospholipase A2 (PLA2) is one of the key enzymes involved in the formation of inflammatory mediators. Inhibition of PLA2 is considered to be one of the efficient methods to control inflammation. In silico docking studies of 160 selected indole derivatives performed against porcine pancreatic PLA2 (ppsPLA2) suggested that, CID2324681, CID8617 (indolebutyric acid or IBA), CID22097771 and CID802 (indoleacetic acid or IAA) exhibited highest binding energies. In silico analysis was carried out to predict some of the ADME properties. The binding potential of these compounds with human non pancreatic secretory PLA2 (hnpsPLA2) was determined using molecular docking studies. In order to corroborate the in silico results, enzyme kinetics and isothermal titration calorimetric analysis of the two selected compounds, IAA and IBA were performed against ppsPLA2. From the analysis, it was concluded that IAA and IBA can act as competitive inhibitors to the enzyme and may be used as anti inflammatory agents.

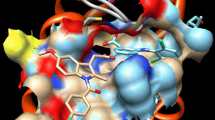
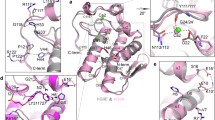
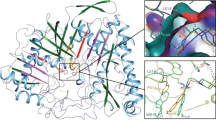
Inhibitory activity of IAA and IBA against PLA2



Similar content being viewed by others
References
Waite M (1987) The phospholipases. In: Hanahan DJ (ed) Handbook of Lipid Research. Plenum, New York
Cifone MG, Botti D, Festuccia C, Napolitano T, del Grosso E, Cavallo G, Chessa MA, Santoni A (1993) Involvement of phospholipase A2 activation and arachidonic acid metabolism in the cytotoxic functions of rat NK cells. Cell Immunol 148:247–258
Pruxanski W, Vadas P (1995) Tenidap sodium inhibits secretory non-pancreatic phospholipase A2 synthesis by foetal rat calvarial osteoblasts. Mediators Inflamm 4:67–70
Needleman P, Turk J, Jakschik BA, Morrison AR, Lefkowith JB (1986) Arachidonic acid metabolism. Annu Rev Biochemi 55:69–102
Ruth JM, Lisa AM (1998) The therapeutic potential for phospholipase A2 inhibitors. Expet Opin Emerg Drugs 3:333–344
Janssen MJ, van de Wiel WA, Beiboer SH, van Kampen MD, Verheij HM, Slotboom AJ, Egmond MR (1999) Catalytic role of the active site histidine of porcine pancreatic phoshpolipase A2 probed by the variants H48Q, H48N and H48K. Protein Eng 12:497–503
Yu BZ, Berg OG, Jam MK (1993) The divalent cation is obligatory for the binding of ligands to the catalytic site of secretory phospholipases A2. Biochemistry 32:6485–6492
Chandra V, Jasti J, Kaur P, Srinivasan A, Betzel C, Singh TP (2002) Structural basis of phospholipase A2 inhibition for the synthesis of prostaglandins by the plant alkaloid aristolochic acid from a 1.7 A crystal structure. Biochemistry 41:10914–10919
Singh N, Jabeen T, Pal A, Sharma S, Perbandt M, Betzel C, Singh TP (2006) Crystal structures of the complexes of a group IIA phospholipase A2 with two natural anti-inflammatory agents, anisic acid, and atropine reveal a similar mode of binding. Proteins 64:89–100
Singh N, Pal A, Jabeen T, Sharma S, Perbandt M, Betzel C, Singh TP Crystal structure of phospholipase A2 in complex with atropine at 1.23A resolution DOI:10.2210/pdb1th6/pdb
Chandra DN, Prasanth GK, Singh N, Kumar S, Jithesh O, Sadasivan C, Sharma S, Singh TP, Haridas M (2011) Identification of a novel and potent inhibitor of phospholipase A2 in a medicinal plant: crystal structure at 1.93 Å and surface plasmon resonance analysis of phospholipase A2 complexed with berberine. Biochim Biophys Acta Protein Proteomics 1814:657–663
Dileep KV, Tintu I, Mandal PK, Karthe P, Haridas M, Sadasivan C (2012) Binding to PLA2 may contribute to the Anti-inflammatory activity of catechol. Chem Biol Drug Des 79:143–147
Schevitz RW, Bach NJ, Carlson DG, Chirgadze NY, Clawson DK, Dillard RD, Draheim SE, Hartley LW, Jones ND, Mihelich ED, Olkowski JL, Snyder DW, Sommers C, Wery JP (1995) Structure-based design of the first potent and selective inhibitor of human non-pancreatic secretory phospholipase A2. Nat Struct Mol Biol 2:458–465
Dillard RD, Bach NJ, Draheim SE, Berry DR, Carlson DG, Chirgadze NY, Clawson DK, Hartley LW, Johnson LM, Jones ND, McKinney ER, Mihelich ED, Olkowski JL, Schevitz RW, Smith AC, Snyder DW, Sommers CD, Wery JP (1996) Indole inhibitors of human nonpancreatic secretory phospholipase A2. 3. Indole-3- acetamides. J Med Chem 39:5119–5136
Draheim SE, Bach NJ, Dillard RD, Berry DR, Carlson DG, Chirgadze NY, Clawson DK, Hartley LW, Johnson LM, Jones ND, McKinney ER, Mihelich ED, Olkowski JL, Schevitz RW, Smith AC, Snyder DW, Sommers CD, Wery JP (1996) Indole inhibitors of human nonpancreatic secretory phospholipase A2. 3. Indole-3-glyoxamides. J Med Chem 39:5159–5175
Hagishita S, Yamada M, Shirahase K, Okada T, Murakami Y, Ito Y, Matsuura T, Wada M, Kato T, Ueno M, Chikazawa Y, Yamada K, Ono T, Teshirogi I, Ohtani M (1996) Potent inhibitors of secretory phospholipase A2: synthesis and inhibitory activities of indolizine and indene derivatives. J Med Chem 39:3636–3658
Yokota Y, Hanasaki K, Ono T, Nakazato H, Kobayashi T, Arita H (1999) Suppression of murine endotoxic shock by sPLA2 inhibitor, indoxam, through group IIA sPLA2-independent mechanisms. Biochim Biophys Acta 1438:213–222
Mouchlis VD, Mavromoustakos TM, Kokotos GJ (2010) Molecular docking and 3D-QSAR COMFA studies on indole inhibitors of GIIA secreted phospholipase A2. Chem Inf Model 50:1589–1601
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Del Rev 46:3–26
Halgren TA (1996) Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J Comp Chem 17:490–519
Schevitz RW, Bach NJ, Carlson DG, Chirgadze NY, Clawson DK, Dillard RD, Draheim SE, Hartley LW, Jones ND, Mihelich ED (1995) Structure-based design of the first potent and selective inhibitor of human non-pancreatic secretory phospholipase A2. Nat Struct Biol 2:458–465
Jorgensen WL, Maxwell DS, Tirado RJ (1996) Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J Am Chem Soc 118:11225–11236
Kaminski GA, Friesner RA, Tirado RJ, Jorgensen WL (2001) Evaluation and reparametrization of the OPLS-AA force field for proteins via comparison with accurate quantum chemical calculations on peptides. J Phys Chem B 105:6474–6487
Levick S, Loch D, Rolfe B, Reid RC, Fairlie DP, Taylor SM, Brown L (2006) Antifibrotic activity of an inhibitor of group IIA secretory phospholipase A2 in young spontaneously hypertensive rats. J Immunol 176:7000–7007
Gregory LS, Kelly WL, Reid RC, Fairlie DP, Forwood MR (2006) Inhibitors of cyclooxygenase-2 and secretory phospholipase A2 preserve bone architecture following ovariectomy in adult rats. Bone 39:134–142
Antonopoulou G, Barbayianni E, Magrioti V, Cotton N, Stephens D, Constantinou-Kokotou V, Dennis EA, Kokotos G (2008) Structure-activity relationships of natural and nonnatural amino acidbased amide and 2-oxoamide inhibitors of human phospholipase A(2) enzymes. Bioorg Med Chem 16:10257–10269
Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, Egger M, Jüni P (2011) Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ 342:c7086
Baruth H et al. (1986) Anti-inflammatory and anti-rheumatic drugs. In: Rainsford KD (ed) Newer anti-inflammatory drugs, vol II. CRC, Boca Raton, p 33
Adams SS (1999) Ibuprofen, the propionics and NSAIDs: personal reflections over four decades. Inflammopharmacology 3:191–197
Dulin JN, Moore ML, Grill RJ Jr (2012) The dual COX/5-LOX inhibitor licofelone attenuates P-glycoprotein-mediated drug resistance in the injured spinal cord. J Neurotrauma doi:10.1089/neu.2012.2587
Kulkarni SK, Mehta AK, Kunchandy J (1986) Anti-inflammatory actions of clonidine, guanfacine and B-HT 920 against various inflammagen-induced acute paw oedema in rats. Arch Int Pharmacodyn Ther 279:324–334
Acknowledgments
The authors gratefully acknowledge the ‘Bioinformatics Infrastructure Facility’ (supported by DBT, Govt. of India) located at the department of Biotechnology and Microbiology, Kannur University for providing the computational work. K.V.D. thanks for Indian Council of Medical Research (ICMR) for the Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dileep, K.V., Remya, C., Tintu, I. et al. Interactions of selected indole derivatives with phospholipase A2: in silico and in vitro analysis. J Mol Model 19, 1811–1817 (2013). https://doi.org/10.1007/s00894-012-1741-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1741-4