Abstract
In this work, computations of density functional theory (DFT) were carried out to investigate the nature of interactions in solid 2,6-dibromo-4-nitroaniline (DBNA). This system was selected to mimic the hydrogen/halogen bonding found within crystal structures as well as within biological molecules. DFT (M06-2X/6-311++G**) calculations indicated that the binding energies for different of interactions lie in the range between −1.66 and −9.77 kcal mol−1. The quantum theory of atoms in molecules (QTAIM) was applied to provide more insight into the nature of these interactions. Symmetry-adapted perturbation theory (SAPT) analysis indicated that stability of the Br···Br halogen bonds is predicted to be attributable mainly to dispersion, while electrostatic forces, which have been widely believed to be responsible for these types of interactions, play a smaller role. Our results indicate that, for those nuclei participating in hydrogen/halogen bonding interactions, nuclear quadrupole resonance parameters exhibit considerable changes on going from the isolated molecule model to crystalline DBNA.

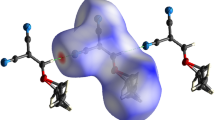
Electrostatic potential mapped on the surface of 2,6-dibromo-4-nitroaniline (DBNA) molecular electron density (0.001 e au−3). Color ranges for V S(r), in kcal mol−1: red > 26.5, yellow 26.5–5.7, green 5.7– −15.1, blue < −15.1. Black circles Surface maxima, blue surface minima



Similar content being viewed by others
References
Liu X, Kwan ICM, Wang S, Wu G (2006) G-quartet formation from an N2-modified guanosine derivative. Org Lett 8:3685–3688
Kouvatsos N, Meldrum JK, Searle MS, Thomas NR (2006) Coupling ligand recognition to protein folding in an engineered variant of rabbit ileal lipid binding protein. Chem Commun 4623–4625
Čern J, Hobza P (2007) Non-covalent interactions in biomacromolecules. Phys Chem Chem Phys 9:5291–5303
Bouchmella K, Dutremez SG, Guérin C, Longato JC, Dahan F (2010) Guanidinium alkynesulfonates with single-layer stacking motif: Interlayer hydrogen bonding between sulfonate anions changes the orientation of the organosulfonate R group from “alternate side” to “same side”. Chem Eur J 16:2528–2536
Scheiner S (1997) Hydrogen bonding: a theoretical perspective. Oxford University Press, New York
Esrafili M, Behzadi H, Hadipour NL (2008) Density functional theory study of N–H⋯O, O–H⋯O and C–H⋯O hydrogen-bonding effects on the 14N and 2H nuclear quadrupole coupling tensors of N-acetyl-valine. Biophys Chem 133:11–18
Behzadi H, Esrafili MD, Hadipour NL (2007) A theoretical study of 17O, 14N and 2H nuclear quadrupole coupling tensors in the real crystalline structure of acetaminophen. Chem Phys 333:97–104
Grabowski SJ, Sokalski WA, Dyguda E, Leszczyńki J (2006) Quantitative classification of covalent and noncovalent H-bonds. Phys Chem B 110:6444–6446
Grabowski SJ (2011) What is the covalency of hydrogen bonding? Chem Rev 111:2597–2625
Desiraju GR, Steiner T (1999) The weak hydrogen bond in structural chemistry and biology. Oxford University Press, New York
Politzer P, Murray JS, Concha MC (2007) Halogen bonding and the design of new materials: organic bromides, chlorides and perhaps even fluorides as donors. J Mol Model 13:643–650
Esrafili MD, Hadipour NL (2011) Characteristics and nature of halogen bonds in linear clusters of NCX (X=Cl, and Br): an ab initio, NBO and QTAIM study. Mol Phys 109:2451–2460
Duarte DJR, de las Vallejos MM, Peruchena NM (2010) Topological analysis of aromatic halogen/hydrogen bonds by electron charge density and electrostatic potentials. J Mol Model 16:737–748
Palusiak M (2010) On the nature of halogen bond—the Kohn–Sham molecular orbital approach. J Mol Struct (THEOCHEM) 945:89–92
Bernard-Houplain M-C, Sandorfy C (1973) Low temperature infrared study of hydrogen bonding in dissolved pyrrole and indole. Can J Chem 51:1075–1082
Bernard-Houplain M-C, Sandorfy C (1973) A low temperature infrared study of hydrogen bonding in N-Alkylacetamides. Can J Chem 51:3640–3646
Rissanen K (2008) Halogen bonded supramolecular complexes and networks. Cryst Eng Commun 10:1107–1113
Metrangolo P, Neukirch H, Pilati T, Resnati G (2005) Halogen bonding based recognition processes: a world parallel to hydrogen bonding. Acc Chem Res 38:386–395
Metrangolo P, Resnati G (2008) Halogen bonding: fundamentals and applications. Springer, Berlin
Ghosh M, Meerts IATM, Cook A, Bergman A, Brouwer A, Johnson LN (2000) Structure of human transthyretin complexed with bromophenols: a new mode of binding. Acta Crystallogr Sect D Biol Crystallogr 56:1085–1095
Bertani R, Chaux F, Gleria M, Metrangolo P, Milani R, Pilati T, Resnati G, Sansotera M, Venzo A (2007) Supramolecular rods via halogen bonding-based self-assembly of fluorinated phosphazene nanopillars. Inorg Chim Acta 360:1191–1199
Cariati E, Forni A, Biella S, Metrangolo P, Meyer F, Resnati G, Righetto S, Tordin E, Ugo R (2007) Tuning second-order NLO responses through halogen bonding. Chem Commun 2007:2590–2592
Brinck T, Murray JS, Politzer P (1993) Molecular surface electrostatic potentials and local ionization energies of Group V–VII hydrides and their anions: Relationships for aqueous and gas-phase acidities. Int J Quantum Chem 48:73–88
Auffinger P, Hays FA, Westhof E, Ho PS (2004) Halogen bonds in biological molecules. Proc Natl Acad Sci USA 101:16789–16794
Politzer P, Lane P, Concha MC, Ma YG, Murray JS (2007) An overview of halogen bonding. J Mol Model 13:305–311
Clark T, Hennemann M, Murray JS, Politzer P (2007) Halogen bonding: the σ-hole. J Mol Model 13:291–296
Murray JS, Lane P, Politzer P (2009) Expansion of the σ-hole concept. J Mol Model 15:723–729
Murray-Rust P, Motherwell WDS (1979) Computer retrieval and analysis of molecular geometry. 4. Intermolecular interactions. J Am Chem Soc 101:4374–4376
Murray-Rust P, Stallings WC, Monti CT, Preston RK, Glusker JP (1983) Intermolecular interactions of the carbon-fluorine bond: the crystallographic environment of fluorinated carboxylic acids and related structures. J Am Chem Soc 105:3206–3214
Ramasubbu N, Parthasarathy R, Murray-Rust P (1986) Angular preferences of intermolecular forces around halogen centers: preferred directions of approach of electrophiles and nucleophiles around carbon-halogen bond. J Am Chem Soc 108:4308–4314
Politzer P, Murray JS, Concha MC (2008) σ-hole bonding between like atoms; a fallacy of atomic charges. J Mol Model 14:659–665
Trogdon G, Murray JS, Concha MC, Politzer P (2007) Molecular surface electrostatic potentials and anesthetic activity. J Mol Mod 13:313–318
Awwadi FF, Willett RD, Peterson KA, Twamley B (2006) The nature of halogen···halogen synthons: crystallographic and theoretical studies. Chem Eur J 12:8952–8960
Metrangolo P, Murray JS, Pilati T, Politzer P, Resnati G (2011) The fluorine atom as a halogen bond donor, viz. a positive site. Cryst Eng Comm 13:6593–6596
Metrangolo P, Murray JS, Pilati T, Politzer P, Resnati G, Terraneo G (2011) Fluorine-centered halogen bonding: a factor in recognition phenomena and reactivity. Cryst Growth Des 11:4238–4246
Valerio G, Raos G, Meille SV, Metrangolo P, Resnati G (2000) Halogen bonding in fluoroalkylhalides: a quantum chemical study of increasing fluorine substitution. J Phys Chem A 104:1617–1620
Riley KE, Hobza P (2008) Investigations into the nature of halogen bonding including symmetry adapted perturbation theory analyses. J Chem Theory Comput 4:232–242
Berski S, Ciunik Z, Drabent K, Latajka Z, Panek J (2004) Dominant role of C − Br···N halogen bond in molecular self-organization. Crystallographic and quantum-chemical study of Schiff-base-containing triazoles. J Phys Chem B 108:12327–12332
Esrafili MD, Ahmadi B (2012) A theoretical investigation on the nature of Cl···N and Br···N halogen bonds in F-Ar-X···NCY complexes (X = Cl, Br and Y = H, F, Cl, Br, OH, NH2, CH3 and CN). Comput Theor Chem 997:77–82
Riley KE, Murray JS, Politzer P, Concha MC, Hobza P (2009) Br ···O complexes as probes of factors affecting halogen bonding: interactions of bromobenzenes and bromopyrimidines with acetone. J Chem Theory Comput 5:155–163
Riley KE, Murray JS, Fanfrlík J, Řezáč J, Solá RJ, Concha MC, Ramos FM, Politzer P (2012) Halogen bond tunability II: the varying roles of electrostatic and dispersion contributions to attraction in halogen bonds. J Mol Model. doi:10.1007/s00894-012-1428-x
Esrafili MD (2012) Investigation of H-bonding and halogen-bonding effects in dichloroacetic acid: DFT calculations of NQR parameters and QTAIM analysis. J Mol Model. doi:10.1007/s00894-012-1496-y
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) General Atomic and Molecular Electronic Structure System. J Comput Chem 14:1347–1363
Bryant R, James SC, Norman NC, Orpen AG (1998) 2,6-Dibromo-4-nitroaniline. Acta Cryst C 54:1113–1115
Zhao Y, Schultz NE, Truhlar DG (2005) Exchange-correlation functional with broad accuracy for metallic and nonmetallic compounds, kinetics, and noncovalent interactions. J Chem Phys 123:161103
Zhao Y, Schultz NE, Truhlar DG (2006) Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J Chem Theory Comput 2:364–382
Zhao Y, Truhlar DG (2006) A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J Chem Phys 125:194101
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functional. Theor Chem Acc 120:215–241
Zhao Y, Truhlar DG (2007) Density functionals for noncovalent interaction energies of biological importance. J Chem Theory Comput 3:289–300
Gu JD, Wang J, Leszczynski J, Xie YM, Schaefer HF (2008) To stack or not to stack: performance of a new density functional for the uracil and thymine dimers. Chem Phys Lett 459:164–166
Bulat FA, Toro-Labbe A, Brinck T, Murray JS, Politzer P (2010) Quantitative analysis of molecular surfaces: areas, volumes, electrostatic potentials and average local ionization energies. J Mol Model 16:1679–1691
Bader RFW (1990) Atoms in molecules-a quantum theory. Oxford University Press, New York
Biegler-Konig F, Schonbohm J, Bayles D (2001) AIM 2000. J Comput Chem 22:545–559
Jeziorski B, Moszynski R, Szalewicz K (1994) Perturbation theory approach to intermolecular potential energy surfaces of Van der Waals complexes. Chem Rev 94:1887–1930
Dalton, a molecular electronic structure program, Release 2.0 (2005) http://www.kjemi.uio.no/software/dalton/dalton.html
Bukowski R, Cencek W, Jankowski P, Jeziorski B, Jeziorska M, Kucharski SA, Lotrich VF, Misquitta AJ, Moszynski R, Patkowski K, Podeszwa R, Rybak S, Szalewicz K, Williams HL, Wheatley RJ, Wormer PES, Żuchowski PS (2008) SAPT2008: “An ab initio program for many-body symmetry-adapted perturbation theory calculations of intermolecular interaction energies” by sequential and parallel versions; University of Delaware and University of Warsaw; http://www.physics.udel.edu/_szalewic/SAPT/SAPT.html
Cukras J, Sadlej J (2008) Symmetry-adapted perturbation theory interaction energy decomposition for some noble gas complexes. Chem Phys Lett 459:44–48
Lucken EAC (1990) Nuclear quadrupole coupling constants. Academic, London
Pyykkö P (2001) Spectroscopic nuclear quadrupole moment. Mol Phys 99:1617–1629
Stewart RF (1972) Valence structure from X–ray diffraction data: physical properties. J Chem Phys 57:1664–1668
Hirschfelder JO, Curtiss CF, Bird RB (1954) Molecular theory of gases and liquids. Wiley, New York
Bader RFW, Carroll MT, Cheeseman JR, Chang C (1987) Properties of atoms in molecules: atomic volumes. J Am Chem Soc 109:7968–7979
Bondi A (1964) van der Waals volumes and radii. J Phys Chem 68:441–451
Vener MV, Manaev AV, Egorova AN, Tsirelson VG (2007) QTAIM study of strong H-bonds with the O − H···A fragment (A = O, N) in three-dimensional periodical crystals. J Phys Chem A 111:1155–1162
LaPointe SM, Farrag S, Bohórquez HJ, Boyd RJ (2009) QTAIM study of an α-helix hydrogen bond network. J Phys Chem B 113:10957–10964
Rozas I, Alkorta I, Elguero J (2000) Behaviour of ylides containing N, O and C atoms as hydrogen bond acceptors. J Am Chem Soc 122:11154–11161
Esrafili MD, Beheshtian J, Hadipour NL (2011) Computational study on the characteristics of the interaction in linear urea clusters. Int J Quantum Chem 111:3184–3195
Panek JJ, Jezierska A (2007) Symmetry-adapted perturbation theory analysis of the N···HX hydrogen bonds. J Phys Chem A 111:650–655
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Jaffe RL, Smith GD (1996) A quantum chemistry study of benzene dimer. J Chem Phys 105:2780–2788
Esrafili MD (2012) Characteristics and nature of the intermolecular interactions in boron-bonded complexes with carbene as electron donor: an ab initio, SAPT and QTAIM study. J Mol Model 18:2003–2011
Song H-J, Xiao H-M, Dong H-S (2006) Theoretical study of properties of H-bonds and intermolecular interactions in linear cis-, trans-cyclotriazane clusters (n = 2–8). J Chem Phys 124:74317
Politzer P, Riley KE, Bulat FA, Murray JS (2012) Perspectives on halogen bonding and other r-hole interactions: Lex parsimoniae (Occam’s Razor). Comput Theor Chem 998:2–8
Yim CT, Whitehead MA, Lo DH (1968) The chemical interpretation and the temperature dependence of the 14N nuclear quadrupole resonance of aniline and several derivatives. Can J Chem 46:3594–3604
Rabbani SR, Edmonds DT, Gosling P (1987) Nuclear quadrupole resonance of 14N and 2H in pyrimidines, purines, and their nucleosides. J Magn Reson 72:422–433
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 68 kb)
Rights and permissions
About this article
Cite this article
Esrafili, M.D. A theoretical investigation of the characteristics of hydrogen/halogen bonding interactions in dibromo-nitroaniline. J Mol Model 19, 1417–1427 (2013). https://doi.org/10.1007/s00894-012-1691-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1691-x