Abstract
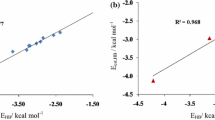
The hydrogen bonding interactions between noradrenaline (NA) and DMSO were studied with density functional theory (DFT) regarding their geometries, energies, vibrational frequencies, and topological features of the electron density. The quantum theory of atoms in molecules (QTAIM) and the natural bond orbital (NBO) analyses were employed to elucidate the hydrogen bonding interaction characteristics in noradrenaline-DMSO complexes. The H-bonds involving the hydroxyls hydrogen in NA and the O atom in DMSO are dominant intermolecular H-bonds and are stronger than other H-bonds involving the methyl hydrogen of DMSO as a H-donor. The weak H-bonds also include a π H-bond which involves the benzene ring as a H-donor or H-acceptor. QTAIM identified the weak H-bonds formed between the methyl hydrogen of DMSO and the N atom in NA in some complexes (AB5, AB6 and AB7), which cannot be further confirmed by NBO and other methods, so there are probably no interactions between hydrogen and nitrogen atoms among these complexes. A good linear relationship between logarithmic electron density (lnρ b ) at the bond critical point (BCP) and structural parameter (δR H···Y) was found. The formations of new H-bonds in some complexes are helpful to strengthen the original intramolecular H-bond, this is attributed to the cooperativity of H-bonds in complexes and can be learned from the structure results and the NBO and QTAIM analyses. Analysis of various physically meaningful contributions arising from the energy decomposition procedures show that the orbital interactions of H-bond is predominant during the formation of the complex, moreover, both the hydrogen bonding interaction and the structural deformation are responsible for the stability of the complexes.

The hydrogen bonding interactions between cysteine and noradrenaline (NA) and DMSO were studied at the ωB97XD/6-311++G(d,p) level regarding their geometries, energies, vibrational frequencies, and topological features of the electron density.



Similar content being viewed by others
References
Song YZ (2007) Theoretical study on the electrochemical behavior of norepinephrine at Nafion multi-walled carbon nanotubes modified pyrolytic graphite electrode. Spectrochim Acta Pt A Mol Biomol Spectrosc 67:1169–1177
Baron R, Zayats M, Willner I (2005) Dopamine-, L-DOPA-, adrenaline-, and noradrenaline- induced growth of Au nanoparticles: assays for the detection of neurotransmitters and of tyrosinase activity. Anal Chem 77:1566–1571
Chen SM, Peng KT (2003) The electrochemical properties of dopamine, epinephrine, norepinephrine, and their electrocatalytic reactions on cobalt(II) hexacyanoferrate films. J Electroanal Chem 547:179–189
Perati PR, Cheng J, Jandik P, Hanko VP (2010) Disposable carbon electrodes for liquid chromatographic detection of catecholamines in blood plasma samples. Electroanalysis 22:325–332
Chen W, Lin XH, Luo HB, Huang LY (2005) Electrocatalytic oxidation and determination of norepinephrine at poly(cresol red) modified glassy carbon electrode. Electroanalysis 17:941–945
Dong H, Wang SH, Liu AH, Galligan JJ, Swain GM (2009) Drug effects on the electrochemical detection of norepinephrine with carbon fiber and diamond microelectrodes. J Electroanal Chem 632:20–29
Luczak T (2009) Electroanalysis of norepinephrine at bare gold electrode pure and modified with gold nanoparticles and s-functionalized self-assembled layers in aqueous solution. Electroanalysis 12:1539–1549
Seol H, Jeong H, Jeon S (2009) A selective determination of norepinephrine on the glassy carbon electrode modified with poly(ethylenedioxypyrrole dicarboxylic acid) nanofibers. J Solid State Electrochem 13:1881–1887
Yao H, Li SG, Tang YH, Chen Y, Chen YZ, Lin XH (2009) Selective oxidation of serotonin and norepinephrine over eriochrome cyanine R film modified glassy carbon electrode. Electrochim Acta 54:4607–4612
Alagona G, Ghio C (2002) Interplay of intra- and intermolecular H-bonds for the addition of a water molecule to the neutral and N-protonated forms of noradrenaline. Int J Quantum Chem 90:641–656
Nagy PI, Alagona G, Ghio C, Takacs-Novak K (2003) Theoretical conformational analysis for neurotransmitters in the gas phase and in aqueous solution. Norepinephrine. J Am Chem Soc 125:2770–2785
Snoek LC, van Mourik T, Carcabal P, Simons JP (2003) Neurotransmitters in the gas phase: hydrated noradrenaline. Phys Chem Chem Phys 5:4519–4526
Snoek LC, Van Mourik T, Simons JP (2003) Neurotransmitters in the gas phase: a computational and spectroscopic study of noradrenaline. Mol Phys 101:1239–1248
Macleod NA, Simons JP (2004) Beta-blocker conformations in the gas phase: 2-phenoxy ethylamine, its hydrated clusters and 3-phenoxy propanolamine. Phys Chem Chem Phys 6:2878–2884
Van Mourik T (2004) The shape of neurotransmitters in the gas phase: a theoretical study of adrenaline, pseudoadrenaline, and hydrated adrenaline. Phys Chem Chem Phys 6:2827–2837
Huang ZG, Dai YM, Yu L (2010) Density functional theory and topological analysis on the hydrogen bonding interactions in N-protonated adrenaline-DMSO complexes. Struct Chem 21:863–872
Yu ZY, Guo DJ, Wang HQ (2004) Theoretical study on the hydrogen bond interaction between adrenaline and dimethyl sulphoxide. Chin J Chem Phys 17:149–154
Huang ZG, Yu L, Dai YM (2010) Density functional theory and topological analysis on the hydrogen bonds in cysteine-propanoic acid complexes. Struct Chem 21:855–862
Huang ZG, Yu L, Dai YM (2010) Combined DFT with NBO and QTAIM studies on the hydrogen bonds in (CH3OH)n (n = 2-8) clusters. Struct Chem 21:565–572
Rappe AK, Bernstein ER (2000) Ab initio calculation of nonbonded interactions: are we there yet? J Phys Chem A 104:6117–6128
Grimme S (2004) Accurate description of van der Waals complexes by density functional theory including empirical corrections. J Comput Chem 25:1463–1473
Schwabe T, Grimme S (2007) Double-hybrid density functionals with long-range dispersion corrections: higher accuracy and extended applicability. Phys Chem Chem Phys 9:3397–3406
Zhao Y, Truhlar DG (2006) Comparative DFT study of van der Waals complexes: Rare-gas dimers, alkaline-earth dimers, zinc dimer, and zinc-rare-gas dimers. J Phys Chem A 110:5121–5129
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Chai JD, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620
Johnson ER, Mackie ID, DiLabio GA (2009) Dispersion interactions in density-functional theory. J Phys Org Chem 22:1127–1135
Huang ZG, Yu L, Dai YM, Wang HK (2010) Hydrogen bonding interactions in cysteine-urea complexes: theoretical studies of structures, properties and topologies. J Mol Struct THEOCHEM 960:98–105
Rao L, Ke HW, Fu G, Xu X, Yan YJ (2009) Performance of several density functional theory methods on describing hydrogen-bond interactions. J Chem Theory Comput 5:86–96
Riley KE, Pitonak M, Cerny J, Hobza P (2010) On the structure and geometry of biomolecular binding motifs (hydrogen-bonding, stacking, X-H···π): WFT and DFT calculations. J Chem Theor Comput 6:66–80
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford
Popelier PLA (2000) Atoms in molecules: an introduction. Prentice Hall, London
Matta CF, BR J (2007) The quantum theory of atoms in molecules: from solid state to DNA and drug design. WILEY-VCH, Weinheim
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Reed AE, Weinhold F, Curtiss LA, Pochatko DJ (1986) Natural bond orbital analysis of molecular interactions: theoretical studies of binary complexes of HF, H2O, NH3, N2, O2, F2, CO, and CO2 with HF, H2O, and NH3. J Chem Phys 84:5687–5705
McLean AD, Chandler GS (1980) Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J Chem Phys 72:5639–5648
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72:650–654
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies: some procedures with reduced errors. Mol Phys 19:553–566
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian Inc, Wallingford
Biegler-König F (2000) AIM2000, 10th edn. University of Applied Sciences, Bielefeld
Benoit DM (2008) Fast vibrational calculation of anharmonic OH-stretch frequencies for two low-energy noradrenaline conformers. J Chem Phys 129:234304
Van Mourik T (2005) On the relative stability of two noradrenaline conformers. Chem Phys Lett 414:364–368
Van Mourik T, Fruchtl HA (2005) The potential energy landscape of noradrenaline: an electronic structure study. Mol Phys 103:1641–1654
Popelier PLA (1998) Characterization of a dihydrogen bond on the basis of the electron density. J Phys Chem A 102:1873–1878
Tian SX (2004) Quantum chemistry studies of glycine-H2O2 complexes. J Phys Chem B 108:20388–20396
Bondi A (1964) van der Waals Volumes and Radii. J Phys Chem 68:441–451
Hannachi Y, Silvi B, Bouteiller Y (1992) Ab initio study of the structure, cooperativity, and vibrational properties of the H2O:(HF)2 hydrogen bonded complex. J Chem Phys 97:1911–1918
Mo O, Yanez M, Elguero J (1992) Cooperative (nonpairwise) effects in water trimers: an ab initio molecular orbital study. J Chem Phys 97:6628–6638
Frank HS, Wen WY (1957) Ion-solvent interaction. Structural aspects of ion-solvent interaction in aqueous solutions: a suggested picture of water structure. Discuss Faraday Soc 24:133–140
Murray JS, Concha MC, Lane P, Hobza P, Politzer P (2008) Blue shifts vs red shifts in sigma-hole bonding. J Mol Model 14:699–704
Clark T, Hennemann M, Murray JS, Politzer P (2007) Halogen bonding: the sigma-hole. J Mol Model 13:291–296
Murray JS, Lane P, Clark T, Politzer P (2007) Sigma-hole bonding: molecules containing group VI atoms. J Mol Model 13:1033–1038
Murray JS, Lane P, Politzer P (2007) A predicted new type of directional noncovalent interaction. Int J Quantum Chem 107(12):2286–2292
Murray JS, Lane P, Politzer P (2008) Simultaneous α-Hole and hydrogen bonding by sulfur- and selenium-containing heterocycles. Int J Quantum Chem 108:2770–2781
Murray JS, Lane P, Politzer P (2009) Expansion of the sigma-hole concept. J Mol Model 15:723–729
Politzer P, Murray JS, Concha MC (2008) Sigma-hole bonding between like atoms; a fallacy of atomic charges. J Mol Model 14:659–665
Politzer P, Murray JS, Lane P (2007) Sigma-hole bonding and hydrogen bonding: competitive interactions. Int J Quantum Chem 107:3046–3052
Clark T, Murray JS, Lane P, Politzer P (2008) Why are dimethyl sulfoxide and dimethyl sulfone such good solvents? J Mol Model 14:689–697
Acknowledgments
This work is supported by Tianjin Science and Technology Development Fund Projects in Colleges and Universities (No. 20080504).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, Z., Dai, Y., Yu, L. et al. Hydrogen bonding interactions in noradrenaline-DMSO complexes: DFT and QTAIM studies of structure, properties and topology. J Mol Model 17, 2609–2621 (2011). https://doi.org/10.1007/s00894-011-0956-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-011-0956-0