Abstract
Converging evidence has revealed disturbances in the corticostriatolimic system are associated with suicidal behaviors in adults with major depressive disorder. However, the neurobiological mechanism that confers suicidal vulnerability in depressed adolescents is largely unknown. A total of 86 depressed adolescents with and without prior suicide attempts (SA) and 47 healthy controls underwent resting-state functional imaging (R-fMRI) scans. The dynamic amplitude of low-frequency fluctuations (dALFF) was measured using sliding window approach. We identified SA-related alterations in dALFF variability primarily in the left middle temporal gyrus, inferior frontal gyrus, middle frontal gyrus (MFG), superior frontal gyrus (SFG), right SFG, supplementary motor area (SMA) and insula in depressed adolescents. Notably, dALFF variability in the left MFG and SMA was higher in depressed adolescents with recurrent suicide attempts than in those with a single suicide attempt. Moreover, dALFF variability was capable of generating better diagnostic and prediction models for suicidality than static ALFF. Our findings suggest that alterations in brain dynamics in regions involved in emotional processing, decision-making and response inhibition are associated with an increased risk of suicidal behaviors in depressed adolescents. Furthermore, dALFF variability could serve as a sensitive biomarker for revealing the neurobiological mechanisms underlying suicidal vulnerability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Suicide is among the top leading causes of death in young people aged 15–29 years [1]. Globally, over 150,000 suicide deaths were estimated to have occurred in this age group in 2019 [1]. Suicide attempts are even more common than completed suicides [2]. The ratio of attempted suicides to completed suicides is estimated to be 50:1 to 100:1 among adolescents [3].
Major depression disorder (MDD) is the most common cause of suicide death worldwide [4, 5]. The mortality risk for suicide in young depressed patients is over 20-fold higher than that in the general population [6]. Currently, we have a limited understanding of the neurobiological mechanism underlying suicidal vulnerability in depressed adolescents [7]. In addition, suicide risk assessment still relies on a patient’s voluntary self-reporting and a clinician’s judgment [8] that lacks predictive accuracy. Elucidating the neurological basis of suicidal behaviors in MDD during adolescence would be of great significance for early intervention and treatment, because of the structural and functional brain changes and prominent psychosocial development that occur in this period [9,10,11], thus facilitating the development of more objective, targeted and effective strategies for preventing suicide.
Functional magnetic resonance imaging (fMRI) studies hold promise for yielding potential markers of suicidal risk by identifying the neurobiological underpinnings of pathophysiologic mechanisms that are not visible at the behavioral level [11]. Thus far, the majority of fMRI studies have focused on adults, with evidence converging on the implication of the corticostriatolimic system that subserves emotion and impulsivity regulation in suicidal behaviors [7, 12,13,14]; however, functional neuroimaging studies on adolescent suicidal behavior are scarce [7, 11], with Limited task fMRI studies showing abnormal functioning of the salience network, attention network and ventral frontal-limbic system, and resting-state fMRI (R-fMRI) studies identifying altered spontaneous brain activities in the left cerebellum, default-mode network [15] and left prefrontal cortex [16] and decreased internetwork connectivity [17] in juvenile depression with suicide behaviors. However, these conventional functional neuroimaging findings relied on the implicit assumption that brain activity remains stationary during the entire fMRI scan, which may be too simplistic to capture the overall picture because the human brain is a highly dynamic system [18,19,20].
Brain dynamics, which are characterized by temporal variability and flexibility of brain activities, provide information on the variability in the strength or spatial dynamic organization beyond static perspectives [20,21,22]. Recent studies have associated dynamic properties of brain activity with the suicidality of MDD [22,23,24]. Specifically, lower temporal dynamics in brain regions involved in executive and emotional processing have been associated with suicidal ideation in MDD patients [22]. Moreover, combining static and dynamic connectomics could differentiate between depressed patients with and without suicidal ideation [24]. Nevertheless, these studies were all conducted with adults. To our knowledge, previous studies have not investigated the dynamic characteristics of suicidal behaviors in depressed adolescents. Given the immaturity of the involved brain systems in adolescents [7], it is necessary to explore the unique characteristics of the brain dynamics in this age group.
Local brain activities are reflections of the intrinsic brain fluctuations generated from mental and cognitive processes [25,26,27]. Combining an effective metric, namely, the amplitude of low-frequency fluctuation (ALFF) [28], a sliding-window approach can be used to measure time-varying brain activities (dynamic ALFF, dALFF) as the variance of ALFF over time (dALFF variability). The current study used the dALFF method to investigate the neurobiological mechanism that confers suicidal vulnerability in depressed adolescents. Based on the aforementioned studies, we sought to test the following hypotheses: (1) The time-varying pattern of brain activities may be altered in depressed adolescents with suicide attempts; and (2) Altered dALFF variability is correlated with clinical variables and can constitute a potential neuromarker for classifying depressed adolescents with and without suicide attempts as well as for predicting the severity of suicidal ideation. In addition, we also performed an exploratory analysis to identify the neural substrate differences between MDD with single SA (MDD-SSA) and MDD with recurrent SA (MDD-RSA).
Materials and methods
Participants
The current study included 86 treatment-seeking depressed adolescents recruited from the Affiliated Brain Hospital of Guangzhou Medical University and 47 healthy controls (HCs) recruited through community postings. All participants were of Han Chinese ethnicity and right handed, and the age range was from 10 to 17 years.
Depressed adolescents were eligible if they (1) met a diagnosis of first-episode major depressive disorder (MDD) based on DSM-V criteria and (2) were drug-naive or consecutively used psychotropic medication for less than 14 days [29]. Healthy adolescents were eligible if they had no past or current psychiatric diagnoses, and they were matched with the MDD group in terms of age and education. The exclusion criteria for both groups included psychoactive substance abuse, neurological illness, a history of loss of consciousness (≥ 5 min) caused by brain injury, severe general physical illness, MRI scanning contraindications, and IQ lower than 70 based on the Wechsler Abbreviated Scale of Intelligence. Healthy adolescents with suicide behaviors or a family history of any psychiatric disorder and MDD patients with developmental disorders (except attention deficit and hyperactivity disorder) were also excluded. Ten adolescents with MDD and two healthy adolescents were excluded for excessive head movements and poor image quality, and the remaining 76 depressed adolescents and 45 HCs were included in the final analyses. The 76 eligible depressed adolescents were further divided into the MDD-SA group (38 individuals with at least one suicide attempt) and the MDD-NSA group (38 individuals without prior suicide attempts). Notably, given that previous studies indicated that the clinical characteristics of single and recurrent attempters were different [30,31,32], we stratified the MDD-SA group into two subgroups, namely, the MDD-SSA (N = 15) and MDD-RSA (N = 19), to explore the underlying neurobiological mechanism differences between them (the total number of suicide attempts of four participants was missing in our dataset).
Written informed consent and assent were obtained from all participants and their parents prior to participation. The current study was part of the work of a multidimensional cohort study on the Symptomatic trajectory and Biomarkers of Early Adolescent Depression (sBEAD), of which the study protocol has been published before (Chinese Clinical Trial Registry Identifier: ChiCTR2100049066) [33]. The institutional review board of Affiliated Brain Hospital of Guangzhou Medical University approved the study protocol, and this study was conducted in accordance with the amended principles of the Declaration of Helsinki.
Clinical assessment
All participants underwent clinical evaluations using the Schedule for Affective Disorders and Schizophrenia for School-Aged Children (K-SADS-PL) matched for DSM-V by qualified psychiatrists to screen for diagnosis.
The lifetime history of suicidal behaviors was assessed using the Columbia Suicide Severity Rating Scale (C-SSRS). The C-SSRS has demonstrated good convergent, divergent, predictive validity in adolescent samples [34, 35]. We assessed suicidal ideation (SI) severity for each participant once the SI severity was endorsed. We focused on the baseline C-SSRS lifetime SI intensity score because it is a significant predictor of subsequent suicide attempts during the 6 month follow-up period [36]. The SI intensity score was derived from five rating items: frequency, duration, controllability, deterrents and reasons for ideation [36, 37]. Suicide attempts were defined as self-injurious behaviors with any intention to die [38, 39]. It should be noted that suicide attempt in our study was referred to an actual suicide attempt, ambiguous attempts such as interrupted, aborted suicide attempt, preparatory acts toward attempt, or non-suicidal self-harm were not included in SA group. The number and methods of suicide attempts, medical lethality and trigger events were also recorded. The severity of depression and anxiety was evaluated with the 24-item Hamilton Depression Scale (HAMD) and Hamilton Anxiety Scale (HAMA).
MRI acquisition
All participants were scanned during the eyes-closed rest condition on a 3.0 Tesla MRI system (Achieva X-series, Philips Medical Systems, Best, Netherlands) in the Department of Radiology at The Affiliated Brain Hospital of Guangzhou Medical University. Foam pads and headphones were used to minimize head motion and reduce scanner noise. Participants were not allowed to take medicine or drink stimulating beverages before the day of scanning. None of the subjects fell asleep during the scan, which was confirmed by a questionnaire following the scan. R-fMRI data were acquired using a gradient-echo echo-planar imaging sequence with the following parameters: repetition time (TR) = 2000 ms, TE = 30 ms, flip angle (FA) = 90°, field of view (FOV) = 220 × 220 mm2, matrix = 64 × 64, axial slices = 33, thickness = 4 mm, gap = 0.6 mm, and voxel size = 3.44 × 3.44 × 4 mm3. The scan lasted for 8 min 12 s (240 volumes). Three-dimensional T1-weighted images were obtained using a fast gradient echo sequence with the following parameters: TR/TE = 8.2 ms/3.8 ms, FOV = 256 × 256 mm2, matrix = 256 × 256, sagittal slices = 188, thickness = 1 mm, and voxel size = 1 × 1 × 1 mm3. Axial T2-weighted images were also collected to exclude anatomical abnormalities.
All functional imaging data preprocessing was performed using the Data Processing and Analysis for Brain Imaging (DPABI V4.3, http://rfmri.org/dpabi) and Statistical Parametric Mapping (SPM12, http://www.fil.ion.ucl.ac.uk/spm) software packages. Preprocessing steps included discarding the first 10 volume images and performing slice timing correction, head motion correction, normalization of the functional images to the Montreal Neurological Institute (MNI) space using unified segmentation of the T1-weighted images with 3 × 3 × 3 mm3 resampling, spatial smoothing with a 4 mm Gaussian kernel, linear detrending, and regression of confounding covariates (Friston-24 motion parameters, white matter, cerebrospinal fluid). As previous studies reported that global signal regression can introduce spurious anti-correlations and substantially alter interregional correlations, we did not remove global signal in the preprocessing procedure [40, 41]. The mean framewise displacement (FD) [42] was calculated to evaluate the head movement of each participant. Subjects were excluded under a head motion criterion of 2.5 mm translation and 2.5° rotation as well as a mean FD of 0.25 mm.
Dynamic ALFF computing
Dynamic ALFF was measured with a sliding-window approach using temporal dynamic analysis (TDA) toolkits based on DPABI. Window size is a controversial parameter for dynamic analysis. Previous studies revealed the window size in the range of 30 s to 1 min was suitable for capturing brain dynamics [43,44,45]; however, another study held different opinions that that the minimum window length should be no less than 1/fmin (1/0.01 = 100 s) [46]. The present study chose 30 TRs (60 s) as window size and we also performed auxiliary analyses with window sizes of 50 TRs (100 s) and 40 TRs (80 s), respectively (Results are provided in supplementary materials). Hamming window was applied to shift the BOLD signals with a window step of 1 TR (2 s). Based on this, the full-length time series of each subject were divided into 201 windows, and the ALFF maps were calculated within each window at the frequency range of 0.01–0.08 Hz, thus generating a set of ALFF maps for each individual. To evaluate the temporal variability (dALFF variability), we then computed the standard deviation (SD) of these dALFF maps across windows. Finally, for standard purposes, the dALFF variability of each voxel was further transformed into z-scores by subtracting the mean and dividing by the SD of global values within a grey mask. To verify whether dALFF and static ALFF (sALFF) shared similar patterns or provided complementary information for uncovering the neurobiology conferring suicidality to depressed adolescents, we examined the sALFF map for each subject as well.
Statistical analysis
Demographic and clinical characteristics
Differences in demographic and clinical characteristics were assessed using SPSS 22.0 software (SPSS Inc.; Chicago, IL, USA). One-way analysis of variance, χ2 tests, two-sample t-tests or Mann–Whitney U test were utilized when appropriate, and significance was set to P < 0.05.
Between-group differences of dALFF variability
The maps of dALFF variability were averaged across all the participants to explore the distribution patterns of dynamic local brain activities within each group. A one-way ANOVA was performed to compare the group differences in dALFF variability among the MDD-SA, MDD-NSA and HC groups, with age, sex, education level and mean FD as covariates. Post hoc analyses were also implemented to assess the dALFF variability differences between each pair of groups within a mask that included all the significant clusters from the ANOVA. Multiple comparisons were performed using Gaussian random field (GRF) theory (voxel-level P < 0.01, cluster-level P < 0.05, one tail for ANOVA and two-tailed for two-sample t-tests). Finally, a two-sample t-test was run again to evaluate the dALFF variability differences between the MDD-RSA and MDD-SSA groups within clusters with significant differences between the MDD-SA group and the other two groups.
Correlation analysis
A partial correlation analysis was conducted to explore the relationship between mean dALFF variability within clusters with significant differences between the MDD-SA and MDD-NSA groups and clinical variables after controlling for confounders, including age, sex, education level and mean FD. The significance level was set to a Bonferroni-corrected P < 0.05/12.
Classification analysis based on dALFF variability
We combined Logistic regression and receiver operating characteristic (ROC) curve analyses, to test the classification performance of dALFF variability in distinguishing MDD-SA from MDD-NSA. We chose mean dALFF attributes that had significant correlations with the SI intensity scores and mean dALFF attributes of all significant clusters as classification features, respectively. Similar classification analyses were also run for sALFF. Finally, we combined all the mean dALFF and sALFF attributes of significant clusters to test whether they can improve the classification power.
Predicting SI intensity based on dALFF variability
To further investigate the relationship between dALFF variability and SI symptoms measured by the C-SSRS. We predicted SI intensity for each individual in the whole patient group using a general linear model. We chose a leave-one-out cross-validation (LOOCV) scheme to it has been demonstrated to be able to yield a robust and reliable model [22, 47]. Specifically, data from a single participant were left out as a test dataset, and the remaining subjects’ data were used as a training set (using the same feature selection strategies as that in the classification analysis) to build a prediction model, based on which the SI intensity scores of the testing dataset were predicted. Finally, Pearson’s correlation analysis was performed to determine whether the predicted SI intensity scores were correlated with the observed SI intensity scores in the MDD group. If a significance level of P < 0.05 was established, we considered that these features could predict SI intensity and vice versa.
Reproducibility validation
We validated our findings of dALFF variability by considering several potential confounding factors. First, to evaluate the influence of sliding window sizes on our results, we conducted auxiliary analyses by recalculating the main results using two other sliding window lengths (40 and 50 TRs). Secondly, as age at onset and illness duration differed between the MDD-SA and MDD-NSA groups, we reperformed a post hoc analysis between these two groups with age at onset and illness duration as additional covariates, respectively.
Results
Demographic and clinical characteristics
As displayed in Table 1, no significant differences in age (F = 1.19, P = 0.308), education level (F = 1.96, P = 0.146) were observed among the three groups, although significant difference were observed in gender (χ2 = 8.91, P = 0.012). Significant differences were not observed in medication counts (χ2 = 0.48, P = 0.923) or in HAMA (T = 1.89, P = 0.063) between the MDD-SA and MDD-NSA groups. In addition, the MDD-SA group scored significantly higher in HAMD (T = 2.41, P = 0.018) and SI intensity (T = 4.29, P < 0.001) than the MDD-NSA group. The age at onset (Z = − 2.88, P = 0.004) of MDD-SA group was earlier than that of the MDD-NSA group, and the illness duration of MDD-SA group was longer than that in MDD-NSA group (Z = − 2.27, P = 0.024). We also observed significant differences of FD values between MDD-SA and MDD-NSA groups (Z = − 2.08, P = 0.040).
Spatial distributing patterns of dALFF variability
MDD-SA, MDD-NSA, and HC displayed similar spatial distribution patterns of dALFF variability, which was nonuniform across the brain. We then performed Pearson's correlation analysis to evaluate the relationship between sALFF and dALFF variability within the MDD-SA, MDD-NSA, and HC groups. The results showed that sALFF metrics were all highly and positively correlated with dALFF variability in these three groups (Figure S2). Brain regions with high temporal variability were mainly distributed in the occipital cortices and postmedial, lateral frontal, parietal, limbic and sensorimotor cortices. Brain regions with low variability located in the inferior temporal and medial prefrontal cortices (Figure S1). The spatial distribution pattern and between-group differences of sALFF maps were highly consistent with that of the dALFF variability (Figure S3).
Between-group differences of dALFF variability
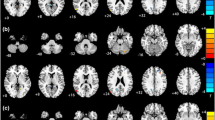
The ANOVA results showed significant dALFF variability differences across the three groups spread over eight clusters, including the right middle temporal pole (MTP)/superior temporal gyrus (STG), left middle temporal gyrus (MTG), left inferior frontal gyrus (IFG) extending to the left insula, left middle frontal gyrus (MFG), left superior frontal gyrus (SFG), right SFG, right supplementary motor area (SMA), and right insula extending to the right putamen (Fig. 1a).
a ANOVA results showing dALFF variability differences across three groups. b–c Post hoc results among the MDD-SA, MDD-NSA, and HC groups. IFG inferior frontal gyrus, INS insula, MFG middle frontal gyrus, SFG superior frontal gyrus, MTG middle temporal gyrus, PUT putamen, MTP middle temporal pole, STG, superior temporal gyrus, SMA supplementary motor area, L left, R right. dALFF dynamic amplitude of low-frequency fluctuations, MDD major depressive disorder, SA prior suicide attempt, NSA no prior suicide attempt, HC healthy control
The post hoc analysis revealed that both the MDD-SA and MDD-NSA groups showed elevated dALFF variability in the left MTG and reduced dALFF variability in the right insula, putamen, SMA and SFG relative to the HC group. In addition, the MDD-SA group exhibited higher dALFF variability in the left MTG and lower dALFF variability in the left IFG, MFG, SFG, right SMA, SMA/SFG and right insula extending to the putamen than the MDD-NSA group (Fig. 1b–c, Table 2).
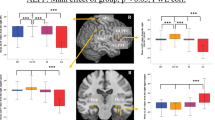
Two-sample t-test revealed that the dALFF variability was higher in the left MFG (T = 2.36, uncorrected P = 0.026) and right SMA (T = 2.30, uncorrected P = 0.028) in the MDD-RSA group than in the MDD-SSA group; however, no cluster survived correction for multiple comparisons (Fig. 2).
dALFF variability difference between MDD-SSA and MDD-RSA in the a left middle frontal gyrus and b right supplementary motor area. * Uncorrected P < 0.05. dALFF dynamic amplitude of low-frequency fluctuations, MFG middle frontal gyrus, SMA supplementary motor area, L left, R right, MDD major depressive disorder, SSA single suicide attempt, RSA recurrent suicide attempts
Correlation analysis
The correlation analysis showed significantly negative correlations between SI intensity scores and dALFF variability values in the left IFG (r = − 0.365, P = 0.0014), right insula (r = − 0.361, P = 0.0016) and SMA/SFG (r = − 0.404, P = 0.0004) in the whole patient group (Fig. 3a–c). The dALFF variability values in the left IFG were negatively correlated with HAMD scores (r = − 0.344, P = 0.0023) (Fig. 3d). No significant correlation between dALFF variability and clinical variables was found within MDD-SA or MDD-NSA group.
Correlation between dALFF variability and clinical variables. a–c dALFF values of the left IFG, right insula and right SMA/SFG were negatively correlated with suicide ideation intensity scores in the whole patient group; d dALFF values of the left IFG were negatively correlated with depression severity evaluated by HAMD in the whole patient group; The shadow indicates 95% confidence intervals. IFG inferior frontal gyrus; INS insula, SMA supplementary motor area, SFG superior frontal gyrus; L left; R right; HAMD Hamilton depression rating Scale; SI suicide ideation; dALFF dynamic amplitude of low-frequency fluctuations
Classification analysis based on dALFF variability
The classification analysis showed that combining the dALFF variability values in the left IFG, right insula and SMA/SFG had the best diagnostic performance (area under the curve (AUC): 0.969, 95% confidence intervals (CI) 0.902–1; sensitivity: 94.44%; specificity: 100%) in discriminating MDD-SA from MDD-NSA, followed by combining the dALFF attributes of all significant clusters (AUC: 0.968, 95% CI 0.900–1; sensitivity: 86.11%; specificity: 97.37%), combining the dALFF and sALFF attributes of all significant clusters (AUC: 0.965, 95% CI 0.900–1; sensitivity: 83.33%; specificity: 100%) and using the sALFF attributes of all significant clusters (AUC: 0.771, 95% CI 0.660–0.869; sensitivity: 80.55%; specificity: 47.36%) (Fig. 4a). Overall, the discriminating power of dALFF variability in differentiating MDD-SA from MDD-NSA was higher than that of sALFF.
Classification and prediction based on dALFF variability and sALFF. a Differentiating MDD-SA from MDD-NSA by selecting different features; (b–c) Predicting SI intensity scores by combining dALFF variability values in the left inferior frontal gyrus, right insula and supplementary motor area/superior frontal gyrus; b and dALFF and sALFF attributes of all significant clusters between the MDD-SA and MDD-NSA groups c. The shadow indicates 95% confidence intervals. dALFF, dynamic amplitude of low-frequency fluctuations; sALFF static amplitude of low-frequency fluctuations; SI suicide ideation
Predicting SI intensity based on dALFF variability
Similar to the classification results, combining the dALFF variability values in the left IFG, right insula and SMA/SFG (r = 0.310, P = 0.007) exhibited the best power in predicting SI intensity, followed by using the dALFF and sALFF attributes of all significant clusters between the MDD-SA and MDD-NSA groups (r = 0.243, P = 0.036); however, using the dALFF attributes (r = 0.138, P = 0.241) and sALFF attributes (r = 0.061, P = 0.607) of all significant clusters could not predict SI intensity (Fig. 4b–c).
Reproducibility validation
Overall, the SA-related alterations in dALFF variability in the different validation strategies were highly consistent with our main results, suggesting a high reproducibility of the results (Figure S3-S7).
Discussion
By using a novel method of exploring brain dynamics, we first identified SA-related alterations in dALFF variability primarily in the left MTG, IFG, MFG, SFG, right SFG, SMA and insula in depressed adolescents. Notably, the altered dALFF variability could be used to generate diagnostic models in discriminating MDD-SA from MDD-NSA with high accuracy, and some features could predict the severity of SI. Moreover, exploratory comparisons also revealed some differences in dALFF variability in the left MFG and right SMA between the MDD-RSA and MDD-SSA groups. Together, these findings provide new insights into the neurobiological mechanisms underlying suicidality in depressed adolescents.
Brain dynamics reflect the functional capacity of the neural system and could serve as a novel neuromarker for various neuropsychiatric disorders [48,49,50], including MDD [22, 51]. Currently, abnormal dynamics of local brain activity [22] and interregional functional connectivity [23, 24] have been demonstrated to be associated with suicidality in adults with MDD. Expanding on previous studies on adults, the current work identified SA-related alterations in dALFF variability in depressed adolescents for the first time.
The IFG (BA 45) and SFG (BA 10), as parts of the ventrolateral prefrontal cortex (VLPFC), play an important role in cognitive control and response inhibition [7], and these behaviors are usually impaired in suicide attempters [52]. The MFG (BA 46) is an integral component of the dorsolateral prefrontal cortex (DLPFC) involved in decision-making over value processing, which is another higher-order cognitive function implicated in suicide diathesis [7, 52, 53]. High IFG and SFG activation have been associated with past suicidal behaviors in psychotic major mood disorders during cognitive control task performance [54]. Similarly, an R-fMRI study found significantly decreased zALFF values in the left SFG and MFG in young depressed patients with suicide behavior [16]. Recent studies have also reported blunted activation of the DLPFC in response to value differences in patients with suicide attempts [55], particularly in well-planned SAs [56]. The SMA (medial portion of BA 6) is one of the key regions that are functionally linked to form the cognitive control loop [57, 58]. Converging neurophysiological and imaging findings indicate that SMA is crucial for correct response inhibition [59, 60]. The functional connectivity between the right SMA and medial frontal cortex had been shown to be significantly correlated with SI intensity in depression [61] as well as past suicide behavior in schizophrenia [57]. Consistent with the limited suicide studies on adolescents, we found lower brain dynamics primarily located in the VLPFC, DLPFC and SMA, which are associated with cognitive control, decision-making and response inhibition. As indicated by previous studies[62, 63], a dynamic brain is important for cognitive functioning while a less dynamic brain activity is related to worse performance on cognitive tasks. Taken together, we speculated that reduced dALFF variability in the VLPFC, DLPFC and SMA may contribute to diminished top-down inhibitory control of behavior and impaired decision-making and planning[7, 64], thus leading to an increased risk of suicide attempts in juvenile depression.
The insula cortex is a key hub of the salience network that mediates or switches between the extended ventromedial prefrontal cortex and dorsal prefrontal cortex/IFG system [65, 66], which possibly facilitates the transition from SI to attempt [7]. Lower insula volume [67, 68] and cortical thickness [69] have been demonstrated in adult SAs across different psychiatric disorders, and altered insula volume was related to attempted lethality and impulsivity [67, 70]. Besides, the disrupted functional connectivity between the insula and SMA was reported to be closely associated with SA in young depressed patients [71]. Furthermore, our previous study showed the functional connectivity strength in the right insula was decreased in patients with bipolar disorder and SA [72]. The underlying biological mechanism that insula affecting suicidal behaviors is still unclear. previous studies indicated that immune challenges activated interoceptive brain pathways (including the insula), triggering alterations in the emotion, motivation and cognition [73]. Additionally, one positron emission tomography study reported that patients with MDD and SI had increased translocator protein activity than those without SI, this result was consistent with postmortem findings of increasing microglia cell activation in MDD with SI, suggesting that the insula may mediate suicidal thoughts and behaviors through neuroinflammation pathway [74]. In line with these findings, the current study suggests that the insula may also underlie the neural mechanism of suicidal behaviors in depressed adolescents.
The temporal association cortices are associated with the perception of intentional behavior and retrieval of personal experiences from memory, and they are also involved in semantic and emotional processing [16, 75]. Consistently, both decreased volume [76] and zALFF values [16] in the MTG were observed in young depressed patients with SA, and the intrinsic functional connectivity strength between the MTG and right anterior cingulate cortex was positively correlated with SI. When viewing 50% intensity angry faces, adolescents with history of suicide attempt and depression showed significantly greater activity in right middle temporal cortex than adolescents with history of depression alone. Additionally, Parkar et al. found individuals with suicidal behaviors had decreased cerebral glucose metabolism in temporal cortex [77]. Consistent with these studies, our study demonstrated elevated dALFF variability in the left MTG, which may underlie emotional dysregulation in youth with depression and suicidal behaviors.
Previous studies indicated that the clinical characteristics of MDD-SSA and MDD-RSA were different [30,31,32, 78], nevertheless, neuroimaging studies have not been performed to investigate the underlying neurobiological mechanism between them. In our exploratory comparison, we found some differences in dALFF variability in the left MFG and right SMA between MDD-RSA and MDD-SSA. However, these two clusters cannot survive multiple comparison correction, which may be related to the small sample size. Given their important roles in decision-making and inhibition control mentioned before, we postulated that the aggravation of disturbance in the left MFG and right SMA may cause impulsive decision-making and incorrect response inhibition, thus leading to an increasing risk of recurrence of suicide attempts.
Limitations
Several limitations of the current study warrant consideration. First, although we believe that our fMRI data could provide adequate information for performing a dynamic analysis, it should be noted that fMRI scans with higher temporal resolution (i.e., multiband technique) would be better for investigating the temporal dynamics of intrinsic brain activities. Second, the LOOCV model used in predicting SI intensity is an unbiased and suitable method for analyzing datasets with small sample sizes; however, it may produce higher variance in prediction errors [79]. Third, although depressed participants received consecutive psychotropic medications for less than two weeks, we could still not completely rule out the possible influence of medications on our results. Fourth, the current study was cross-sectional, and studies with longitudinal designs are essential for identifying the risk markers of future suicide attempts. Last, due to the small size, the results should be interpreted with caution and require replication in further research with a larger sample size.
Conclusions
In summary, the current study identified SA-related alterations in dALFF variability primarily in the left MTG, IFG, MFG, SFG, right SFG, SMA and insula in depressed adolescents. Disturbances of the brain dynamics in these brain regions may contribute to emotional dysregulation, impaired decision-making and incorrect inhibition control, thus leading to an increased risk of suicidal behaviors. Moreover, dALFF variability was capable of generating better diagnostic and prediction models for suicidality than static ALFF. Our findings provide new insights into the neurobiological mechanisms underlying suicidal behaviors in youth with depression.
Data availability
The data that support the findings of this study are available from the corresponding author.
References
WHO, Suicide worldwide in (2019). Global health estimates. https://www.who.int/publications/i/item/suicide-in-the-world. 2019.
Becker M, Correll CU (2020) Suicidality in childhood and adolescence. Dtsch Arztebl Int 117(15):261–267
Shain B (2016) Committee On, suicide and suicide attempts in adolescents. Pediatrics 138(1):e20161420
Bachmann S (2018) Epidemiology of suicide and the psychiatric perspective. Int J Environ Res Public Health 15(7):1425
Ferrari AJ et al (2013) Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med 10(11):e1001547
Lepine JP, Briley M (2011) The increasing burden of depression. Neuropsychiatr Dis Treat 7(Suppl 1):3–7
Schmaal L et al (2020) Imaging suicidal thoughts and behaviors: a comprehensive review of 2 decades of neuroimaging studies. Mol Psychiatry 25(2):408–427
Asarnow JR, Mehlum L (2019) Practitioner review: treatment for suicidal and self-harming adolescents—advances in suicide prevention care. J Child Psychol Psychiatry 60(10):1046–1054
Baum GL et al (2020) Development of structure-function coupling in human brain networks during youth. Proc Natl Acad Sci USA 117(1):771–778
Maalouf FT, Brent DA (2012) Child and adolescent depression intervention overview: what works, for whom and how well? Child Adolesc Psychiatr Clin N Am 21(2):299–312
Martin PC, Zimmer TJ, Pan LA (2015) Magnetic resonance imaging markers of suicide attempt and suicide risk in adolescents. CNS Spectr 20(4):355–358
Alacreu-Crespo A et al (2020) Prefrontal activation in suicide attempters during decision making with emotional feedback. Transl Psychiatry 10(1):313
Du L et al (2017) Fronto-limbic disconnection in depressed patients with suicidal ideation: a resting-state functional connectivity study. J Affect Disord 215:213–217
Malhi GS et al (2019) Cognitive and emotional impairments underpinning suicidal activity in patients with mood disorders: an fMRI study. Acta Psychiatr Scand 139(5):454–463
Shu Y et al (2020) Fractional amplitude of low-frequency fluctuation (fALFF) alterations in young depressed patients with suicide attempts after cognitive behavioral therapy and antidepressant medication cotherapy: a resting-state fMRI study. J Affect Disord 276:822–828
Cao J et al (2016) Resting-state functional MRI of abnormal baseline brain activity in young depressed patients with and without suicidal behavior. J Affect Disord 205:252–263
Cao J et al (2020) Altered resting-state functional network connectivity is associated with suicide attempt in young depressed patients. Psychiatry Res 285:112713
Casorso J et al (2019) Dynamic mode decomposition of resting-state and task fMRI. Neuroimage 194:42–54
Preti MG, Bolton TA, Van De Ville D (2017) The dynamic functional connectome: state-of-the-art and perspectives. Neuroimage 160:41–54
Fu Z et al (2018) Characterizing dynamic amplitude of low-frequency fluctuation and its relationship with dynamic functional connectivity: an application to schizophrenia. Neuroimage 180(Pt B):619–631
Bassett DS, Sporns O (2017) Network neuroscience. Nat Neurosci 20:353–364
Li J et al (2019) More than just statics: temporal dynamics of intrinsic brain activity predicts the suicidal ideation in depressed patients. Psychol Med 49(5):852–860
Qiao D et al (2020) Altered static and dynamic functional connectivity of habenula associated with suicidal ideation in first-episode, drug-naive patients with major depressive disorder. Front Psychiatry 11:608197
Liao W et al (2018) Static and dynamic connectomics differentiate between depressed patients with and without suicidal ideation. Hum Brain Mapp 39(10):4105–4118
Raichle ME, Snyder AZ (2007) A default mode of brain function: a brief history of an evolving idea. Neuroimage 37(4):1083–90
Britz J, Pitts MA, Michel CM (2011) Right parietal brain activity precedes perceptual alternation during binocular rivalry. Hum Brain Mapp 32(9):1432–1442
Hutchison RM, Morton JB (2016) It’s a matter of time: reframing the development of cognitive control as a modification of the brain’s temporal dynamics. Dev Cogn Neurosci 18:70–77
Zang YF et al (2007) Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev 29(2):83–91
Kornstein SG et al (2016) The effects of levomilnacipran ER in adult patients with first-episode, highly recurrent, or chronic MDD. J Affect Disord 193:137–143
Stringer B et al (2013) Recurrent suicide attempts in patients with depressive and anxiety disorders: the role of borderline personality traits. J Affect Disord 151(1):23–30
Miranda R et al (2008) Suicide attempt characteristics, diagnoses, and future attempts: comparing multiple attempters to single attempters and ideators. J Am Acad Child Adolesc Psychiatry 47(1):32–40
Speed KJ, Drapeau CW, Nadorff MR (2018) Differentiating single and multiple suicide attempters: what nightmares can tell us that other predictors cannot. J Clin Sleep Med 14(5):829–834
Zhang X et al (2022) A cohort study of adolescents with depression in China: tracking multidimensional outcomes and early biomarkers for intervention. Gen Psychiatr 35(4):e100782
Posner K et al (2011) The Columbia-suicide severity rating scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 168(12):1266–1277
Huber RS et al (2019) Reduced lateral orbitofrontal cortex volume and suicide behavior in youth with bipolar disorder. Bipolar Disord 21(4):321–329
Lindh AU et al (2018) Short term risk of non-fatal and fatal suicidal behaviours: the predictive validity of the Columbia-suicide severity rating scale in a swedish adult psychiatric population with a recent episode of self-harm. BMC Psychiatry 18(1):319
Lindh AU et al (2019) A comparison of suicide risk scales in predicting repeat suicide attempt and suicide: a clinical cohort study. J Clin Psychiatry. https://doi.org/10.4088/JCP.18m12707
De Crescenzo F et al (2017) Suicide attempts in juvenile bipolar versus major depressive disorders: systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 56(10):825-831.e3
Johnston JAY et al (2017) Multimodal neuroimaging of frontolimbic structure and function associated with suicide attempts in adolescents and young adults with bipolar disorder. Am J Psychiatry 174(7):667–675
Murphy K, Fox MD (2017) Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage 154:169–173
Saad ZS et al (2012) Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect 2(1):25–32
Jenkinson M et al (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17(2):825–841
Fu Z et al (2018) Characterizing dynamic amplitude of low-frequency fluctuation and its relationship with dynamic funct ional connectivity: an application to schizophrenia. Neuroimage 180(Pt B):619–631
de Lacy N et al (2017) Disruption to control network function correlates with altered dynamic connectivity in the wider autism spectrum. Neuroimage Clin 15:513–524
Rashid B et al (2014) Dynamic connectivity states estimated from resting fMRI Identify differences among Schizophrenia, bipolar disorder, and healthy control subjects. Front Hum Neurosci 8:897
Liao W et al (2014) Dynamical intrinsic functional architecture of the brain during absence seizures. Brain Struct Funct 219(6):2001–2015
Finn ES et al (2015) Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci 18(11):1664–1671
Liu F et al (2017) Dynamic functional network connectivity in idiopathic generalized epilepsy with generalized tonic-clonic seizure. Hum Brain Mapp 38(2):957–973
Ma L et al (2021) Dynamic functional connectivity alterations and their associated gene expression pattern in autism spectrum disorders. Front Neurosci 15:794151
Xue K et al (2022) Local dynamic spontaneous brain activity changes in first-episode, treatment-naive patients with major depressive disorder and their associated gene expression profiles. Psychol Med 52(11):2052–2061
Bolton TAW et al (2020) Tapping into multi-faceted human behavior and psychopathology using fMRI brain dynamics. Trends Neurosci 43(9):667–680
Lengvenyte A et al (2021) Biological bases of suicidal behaviours: a narrative review. Eur J Neurosci 53(1):330–351
Tsutsui KI et al (2016) A dynamic code for economic object valuation in prefrontal cortex neurons. Nat Commun 7:12554
Minzenberg MJ et al (2015) Control-related frontal-striatal function is associated with past suicidal ideation and behavior in patients with recent-onset psychotic major mood disorders. J Affect Disord 188:202–209
Olie E et al (2015) Processing of decision-making and social threat in patients with history of suicidal attempt: a neuroimaging replication study. Psychiatry Res 234(3):369–377
Vanyukov PM et al (2016) Paralimbic and lateral prefrontal encoding of reward value during intertemporal choice in attempted suicide. Psychol Med 46(2):381–391
Minzenberg MJ et al (2015) Conflict-related anterior cingulate functional connectivity is associated with past suicidal ideation and behavior in recent-onset schizophrenia. J Psychiatr Res 65:95–101
de la Pena MJ et al (2020) A practical approach to imaging of the supplementary motor area and its subcortical connections. Curr Neurol Neurosci Rep 20(11):50
Aron AR, Robbins TW, Poldrack RA (2014) Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci 18(4):177–185
Gavazzi G et al (2019) Left inferior frontal cortex can compensate the inhibitory functions of right inferior frontal cortex and pre-supplementary motor area. J Neuropsychol 13(3):503–508
Lee S et al (2019) Altered resting-state functional connectivity in depressive disorder patients with suicidal attempts. Neurosci Lett 696:174–178
Zalesky A et al (2014) Time-resolved resting-state brain networks. Proc Natl Acad Sci U S A 111(28):10341–10346
Schumacher J et al (2019) Dysfunctional brain dynamics and their origin in lewy body dementia. Brain 142(6):1767–1782
Dixon ML et al (2017) Emotion and the prefrontal cortex: an integrative review. Psychol Bull 143(10):1033–1081
Goulden N et al (2014) The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage 99:180–190
Zhou Y et al (2018) The hierarchical organization of the default, dorsal attention and salience networks in adolescents and young adults. Cereb Cortex 28(2):726–737
Soloff PH et al (2012) Structural brain abnormalities and suicidal behavior in borderline personality disorder. J Psychiatr Res 46(4):516–525
Giakoumatos CI et al (2013) Are structural brain abnormalities associated with suicidal behavior in patients with psychotic disorders? J Psychiatr Res 47(10):1389–1395
Besteher B et al (2016) Pronounced prefronto-temporal cortical thinning in schizophrenia: neuroanatomical correlate of suicidal behavior? Schizophr Res 176(2–3):151–157
Soloff P, White R, Diwadkar VA (2014) Impulsivity, aggression and brain structure in high and low lethality suicide attempters with borderline personality disorder. Psychiatry Res 222(3):131–139
Hu L et al (2021) The association between insular subdivisions functional connectivity and suicide attempt in adolescents and young adults with major depressive disorder. Brain Topogr 34(3):297–305
Cheng X et al (2020) Alterations in resting-state global brain connectivity in bipolar I disorder patients with prior suicide attempt. Bipolar Disord. https://doi.org/10.1111/bdi.13012
Savitz J, Harrison NA (2018) Interoception and inflammation in psychiatric disorders. Biol Psychiatry Cogn Neurosci Neuroimaging 3(6):514–524
Holmes SE et al (2018) Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: a positron emission tomography study. Biol Psychiatry 83(1):61–69
Gallagher H, Gallagher FC et al (2023) Functional imaging of “theory of mind.” Trends Cognit Sci. https://doi.org/10.1016/S1364-6613(02)00025-6
Peng H et al (2014) Increased suicide attempts in young depressed patients with abnormal temporal-parietal-limbic gray matter volume. J Affect Disord 165:69–73
Parkar S et al (2022) Suicidal behaviour: What’s the brain up to? J Psychiatr Res 146:210–218
Jakobsen IS et al (2011) Differences between youth with a single suicide attempt and repeaters regarding their and their parents history of psychiatric illness. Arch Suicide Res 15(3):265–276
RK. (1995) A study of cross-validation and bootstrap for accuracy estimation and model selection. In Proceedings of the 14th International Joint Conference on ArtificialIn telligence. 1137–1145.
Acknowledgements
Role of Funding Source
This research was supported in part by the STI 2030- Major projects (2022ZD0211700); the Natural Science Foundation of Guangdong Province, China (2021A1515011288, 2023A1515010606); Science and Technology Program of Guangzhou (202102010020); Jointly supported by Guangzhou Science and Technology Bureau, Municipal (enterprise) and Institute (SL2022A03J01490, SL2022AD3J01477); Health Science and Technology Project of Guangzhou (20211A010037, 20201A010030); Guangzhou Municipal Key Discipline in Medicine (2021-2023); Scientific Research Project of Guangdong Traditional Chinese Medicine Bureau (20222180). We thank Mr. Bin Sun from the Affiliated Brain Hospital of Guangzhou Medical University for his support in statistical analysis.
Author information
Authors and Affiliations
Contributions
XHW, LPC gain research fundings. XHW, LPC and XFC contributed to study design, interpretation of the findings and manuscript edits. CXF wrote the initial draft of the manuscript. XHW and LPC supervised the analysis of study data. XFC, JSC, XFZ, JQS, YLZ, RLY, ZYS, PRC, CJY, QXW, TFL and YMC collected the data. XFC, WT, AMC and YYX performed data cleaning and analysis. All authors have contributed to the drafting of its final version, and critical revision of the manuscript for important intellectual content.
Corresponding authors
Ethics declarations
Conflict of interests
None.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheng, X., Chen, J., Zhang, X. et al. Characterizing the temporal dynamics of intrinsic brain activities in depressed adolescents with prior suicide attempts. Eur Child Adolesc Psychiatry 33, 1179–1191 (2024). https://doi.org/10.1007/s00787-023-02242-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-023-02242-4