Abstract
Objectives
Recent evidence suggested a link between periodontitis (PD) and dental caries, but the trends and nature of this association remained unclear. The overall aim of this study was to critically assess the correlation of two disorders.
Methods
A comprehensive search was conducted within the PUBMED and EMBASE databases including grey literatures up to July 5th, 2023. The Newcastle–Ottawa scale was used to qualitatively evaluate the risk of bias.
Results
Overall, 18 studies were included. In terms of caries risk in PD patients, the prevalence of caries was increased by PD (OR = 1.57, 95%CI:1.20–2.07), both in crown (OR = 1.03, 95%CI:1.01–1.05) and root caries (OR = 2.10, 95%CI:1.03–4.29). Odds of caries were also raised by PD severity (OR moderate = 1.38, 95%CI:1.15–1.66; OR severe = 2.14, 95%CI:1.74–2.64). Besides, patients with PD exhibited a higher mean number of decayed, missing and filled teeth (DMFT) and decayed and filled root teeth (DFR) [weighted mean difference (WMD)DMFT = 0.87, 95%CI: -0.03–1.76; WMDDFR = 1.13, 95%CI: 0.48–1.78]. Likewise, patients with caries had an elevated risk of PD (OR = 1.79, 95%CI:1.36–2.35). However, Streptococcus mutans, one of the main pathogens of caries, was negatively correlated with several main pathogens of periodontitis.
Conclusions
This study indicated a positive correlation between dental caries and periodontitis clinically, while the two disease-associated pathogens were antagonistic.
Clinical relevance
Further research, including clinical cohort studies and mechanisms of pathogens interaction is needed on this link for better prevention and treatment of PD and caries. In addition, innovative prevention strategies need to be developed and incorporated in dental practices to prevent these two highly prevalent oral diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontitis (PD) is a chronic multifactorial inflammatory disease caused by dysbiosis, resulting in progressive destruction of the dental surrounding tissues and tooth loss [1]. Its severe form was considered the 6th most prevalent disease of humankind in 2010, which affected 743 million people aged 15 and over worldwide [2, 3]. Dental plaque is an initiating factor that first causes gingivitis if accumulated, resulting in the loss of collagen locally [4]. Destructive periodontitis will occur gradually if the inflammation is not well controlled, which has been associated with dysbiosis where the diversity, richness and relative proportions of species in the subgingival microbiota are altered [5]. While the periodontal diseases are unquestionably initiated by bacteria, it is the individual’s host inflammatory response and other risk factors that ultimately determine the clinical presentation and outcome of the many and varied forms of periodontal disease [6]. The epithelium lining periodontal pockets becomes ulcerated, providing a direct entry point for periodontal bacteria into the systemic circulation. Alternatively, the inflammatory response to periodontal bacteria or their by-products may have indirect systemic effects linking periodontitis to a number of chronic systemic diseases [7], such as diabetes [8], rheumatoid arthritis [9] and Alzheimer's disease [10].
Dental caries, otherwise known as tooth decay, is one of the most prevalent chronic diseases among people worldwide [11]. According to the World Health Organization (WHO) report in 2022, untreated carious lesions (both deciduous and permanent teeth), severe periodontal disease, edentulism and cancer of the lip and oral cavity were the leading causes of oral disease burden [12]. It is caused by cariogenic microorganisms in the dental plaque biofilm, which ferment dietary carbohydrates to produce acid, leading to mineral loss from tooth hard tissues and subsequently the destruction of tooth structures [13]. The interplay between microorganisms, diet and host susceptibility determines whether dental caries will occur [11]. Dental caries in enamel is typically first seen as white spot lesions. The cavity site provides an ecological niche in which plaque organisms gradually adapt to a reduced pH [14], followed by bacteria penetrating further into the tissue at an earlier stage of lesion development [15] and gradually progressing to pulpitis, periapical inflammation, and even tooth loss if not controlled [16].
Most of the time, a homeostatic balance exists between the host and microbial communities. Distinct microenvironments contain unique microbial communities, which are regulated through sophisticated signal systems and by host and environment [5]. The dynamic and polymicrobial oral microbiome is a direct precursor of dental caries and periodontitis, two of the most prevalent microbially induced disorders worldwide. As a community develops, microbial metabolism and by-products of the host immune response can cause changes to the local environment that facilitate the outgrowth or over-representation of microorganisms associated with a dysbiotic state [17]. Mutans streptococci (especially Streptococcus mutans) and Lactobacillus have long been recognized as pathogens that are associated with caries [5], where previous studies supported a significant correlation between their concentration in saliva and proportion in plaque [13]. While the flora imbalance of Porphyromonas gingivalis, Fusobacterium nucleatum, Actinobacillus actinomycetemcomitans and others are tied to the progression of periodontitis [18,19,20]. Due to the distinction of pathogenic bacteria and clinical manifestations, PD and dental caries were often regarded as two independent diseases and studied separately. Nonetheless, the association between PD and dental caries has been debated in recent years, with some studies showing an inverse association and others showing a positive association between these two diseases [21, 22]. However, little was still known about the direction and nature of the association between these two conditions. The overall aim of this study was to conduct a robust critical appraisal of the evidence on the relationship between PD and dental caries, mainly in terms of correlation of the clinical prevalence and the predominant causative organisms between the two diseases.
Method
Search strategy
Studies were selected based on the PECOS question, including observational studies in humans with caries/periodontitis (P—persons) in which periodontitis/caries was present (E—exposure) or absent (C—comparison) to observe the prevalence of caries/periodontitis and the relationship of major pathogenic bacteria (O—outcome). Hence, we addressed several key questions: Is there any association between periodontitis and caries? Is it positive or negative and are there any clinical and bacteriological correlation between these two diseases?
The search string considered alternate terms incorporating several relevant key words and Medical Subject Headings (MeSH). The final Boolean search string was: (periodontal diseases∗ OR periodontitis) AND (dental caries* OR caries) (Appendix S1). The search string was applied from PubMed and EMBASE databases as well as grey literatures until July 5th 2023 to ensure retrieval of a broad scope of literature.
Eligibility criteria
Eligible studies were examined by two authors independently. The final selection was verified by a third author, and disagreements were resolved through discussions.
Inclusion criteria
Dental caries condition in patients with periodontitis.
-
Population: human.
-
Exposure: subjects with periodontitis. The confident case definition for periodontitis were defined as periodontal pocket depth (PPD) ≥ 4 mm, clinical attachment loss (CAL) ≥ 3 mm, and community periodontal index (CPI) ≥ 3 [23]; The non-confident case definition was considered as ‘Alveolar bone loss’ without other measurements of PPD/CAL; Unclear diagnostic criteria for periodontitis.
-
Non-exposure: subjects without periodontitis (with periodontal health or gingivitis).
-
Outcome: the primary outcome was defined as prevalence of dental caries (crown caries or root caries) in individuals with periodontitis, the secondary outcome was mean DMFT (the amount of decayed, missing, filled permanent teeth) or DFR (the amount of decayed, filled root teeth), along with the correlation of pathogenic bacteria related to caries and PD.
-
Study design: case–control or cohort studies.
Periodontitis condition in patients with caries.
-
Population: human.
-
Exposure: subjects with caries, it was defined as DT ≠ 0.
-
Non-exposure: subjects without caries.
-
Outcome: the primary outcome was defined as prevalence of periodontitis in individuals with caries; the secondary outcome was the correlation of pathogenic bacteria related to caries and periodontitis.
-
Study design: case–control or cohort studies.
Exclusion criteria
-
Studies with groups evaluating periodontitis or caries separately, case reports, review or guidelines, no full text available nor English language used.
-
Publications were further excluded if periodontal status was only assessed by tooth loss or gingival appearance.
Quality assessment
Newcastle–Ottawa Scale (NOS) [24] was employed to evaluate the methodological quality of the included studies, which were defined as moderate or high methodological quality with at least five scores.
Data extraction and processing
Data extraction conducted by YX.Li and YG.Xiang was based on a standardized, pre-piloted data extraction form. The extracted information included: authors and year, study design, characteristics of the sample (size, age, location, and study group); evaluation method (periodontitis and caries diagnosis), statistical analysis, the primary outcomes ([RR] or [OR], 95% confidence interval (CI) or those providing absent raw numbers are available for crude calculation) and the secondary outcomes (mean DMFT or DFR, and the association and interaction of pathogenic bacteria associated with caries and periodontitis).
Statistical analysis
The estimates (or adjusted estimates if applicable) and the corresponding 95% confidence interval (CI) between PD and caries were used to calculate the pooled estimates. If no estimates were available in the studies, the numbers of cases (with PD/caries or not) and controls (with PD/caries or not) were used to calculate the pooled estimates. Heterogeneity was evaluated using the Cochrane I2 statistic [25]. Descriptive statistics were performed to summarize the evidence retrieved, checking further for study variations in terms of study characteristics and results. For caries condition in patients with PD, subgroups analyses were performed in periodontitis diagnostic criteria, periodontitis severity, type of caries, age and gender. Similarly, for PD risk in patients with caries, the subgroups analyses were performed in age and gender. Funnel plot was generated to assess publication bias for primary outcome by the visual inspection of asymmetry. Rev Man 5.1 was employed to perform all analyses.
Results
Literature selection
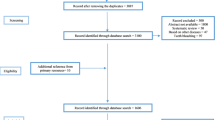
The literature search process was summarized in Fig. 1. Briefly, 10,094 articles were retrieved by an initial database search, including exclusion of 783 duplications. 9,227 publications were excluded after screening the abstracts. 84 publications were eligible for full text screening. Finally, a total of 18 publications were included for final meta-analysis.
Study characteristics and quality assessment
All included studies were published between 1994 and 2022. The characteristics of these 18 studies were shown in Table 1, including 10 studies [26,27,28,29,30,31,32,33,34,35] in relative to the prevalence of caries in patients with periodontitis, 5 [29,30,31,32, 36] containing the prevalence of periodontitis in patients with caries and 7 studies [33, 37,38,39,40,41,42] covering the association between pathogenic bacteria related to two diseases.
Study quality for observational studies assessed by the Newcastle–Ottawa scale varied across the studies, ranging from a score of 5/9 to 8/9 (Appendix S2). The assessment revealed several potential sources of bias including the representativeness of the cases and lack of adjustment for potential confounders.
Dental caries risk in patients with periodontitis
To assess the risk of having caries in individuals with periodontitis, all the 10 relative studies [26,27,28,29,30,31,32,33,34,35] were included. The pooled results showed that the risk of having caries was associated with periodontitis (OR = 1.57, 95% CI: 1.20–2.07, P = 0.001) in Fig. 2.
Subgroup analysis
Definition of periodontitis
Subgroup analysis by the definition of periodontitis indicated that 8 studies [26, 27, 29,30,31,32, 34, 35] with standard definition of periodontitis confirmed higher odds of caries (OR = 1.62, 95% CI: 1.20–2.18; P = 0.002) as well as 2 studies [28, 33] with a non-standard definition of periodontitis. (OR = 1.38, 95% CI: 1.07–1.79; P = 0.01, Fig. 3).
Periodontitis severity
Subgroup analysis by severity of periodontitis demonstrated that caries was significantly associated with moderate (OR = 1.38, 95% CI: 1.15–1.66, P = 0.0006) and severe periodontitis (OR = 2.14, 95% CI: 1.74–2.64, P = 0.0002). One study [29] reported the risk of mild periodontitis with caries (OR = 1.65, 95% CI: 0.45–6.05) in Fig. 4.
Different surfaces of caries
Subgroup analysis by different surfaces of caries showed that the risk of the site where caries occurs varied in crown caries (OR = 1.03, 95% CI: 1.01–1.05, P = 0.003) and root caries (OR = 2.10, 95% CI: 1.03–4.29, P = 0.04). Especially, an increased risk of mixed-type caries defined as covering crown and root surfaces in one study [35] was reported (OR = 3.40, 95% CI: 3.10–3.73, P < 0.00001, Fig. 5).
Age and gender
Additionally, subgroup analyses were also performed by gender and age in Figs. 6 and 7. The risk of caries was both higher in 30 to 64 years old (OR = 1.67, 95% CI: 1.37–2.03, P < 0.00001) and 65 to over 75(OR = 1.69, 95% CI: 1.44–1.98, P < 0.00001). Two studies [27, 32] reported the risk of dental caries between male and female, respectively, which varied in male (OR = 1.48, 95% CI: 1.26–1.74, P < 0.00001) and female (OR = 1.36, 95% CI: 0.74–2.49, P = 0.32).
OR adjusted or not
Subgroup analysis by OR adjusted or not showed that 2 studies [26, 34] reported adjusted ORs, where the precision of the estimate based on the confidence interval bounds was not significant (OR = 1.28, 95% CI: 0.85–1.93, P = 0.24). 8 studies [27,28,29,30,31,32,33, 35] provided absent raw numbers available for crude calculation (OR = 1.69, 95% CI: 1.41–2.01, P = 0.0005, Fig. 8).
Mean DMFT or DFR
Four studies [26, 33, 34, 43] reported mean DMFT or DFR in patients with periodontitis. One study [37] indicated significant positive associations between the periodontal disease severity (CDC-AAP) [23] and the amount of decayed, missing and filled teeth surfaces (DMFS) (OR adjusted = 1.03, 95% CI: 1.01–1.05) and the decayed surfaces (DS) indices (OR adjusted = 1.18, 95% CI: 1.05–1.32) as well as between the percentage of sites with CAL ≥ 3 mm and DMFS (OR adjusted = 1.03, 95% CI: 1.00–1.05) and DS indices (OR adjusted = 1.12, 95% CI: 1.00–1.25). Overall meta-analysis showed Patients with periodontitis exhibited higher DMFT [weighted mean difference (WMD) = 0.87, 95% CI: -0.03–1.76, P = 0.06] and DFR (WMD = 1.13, 95% CI: 0.48–1.78, P = 0.0007) when compared with patients without periodontitis (Appendix S4).
Periodontitis risk in patients with caries
To assess the risk of having periodontitis in individuals with caries, all the 5 relative studies [29,30,31,32, 36] were included. Statistically significant heterogeneity was confirmed with I2 test (I2 = 82%, P = 0.0002). The pooled results in Fig. 9 showed that the risk of periodontitis was associated with caries (OR = 1.79, 95% CI: 1.36–2.35, P = 0.0002), both for crude ORs (2.35, 95% CI: 2.04–2.70, P < 0.00001) and adjusted ORs (1.57, 95%CI: 1.32–1.86, P < 0.00001, Fig. 10). Other subgroups, such as types of caries, severity of periodontitis, age and gender, were not analyzed due to limited data.
Bacterial association between periodontitis and caries
Seven studies [33, 37,38,39,40,41,42] were included as reporting the correlation between the dominant pathogenic bacteria associated with PD and caries. An increased risk of detectable high level (> 104 CFU/ml) of P. gingivalis was reported in the saliva when PD occurred (OR = 3.38, 95% CI: 1.46–7.83, P = 0.005), while S. mutans, one of the main pathogens of caries, showing a declining risk in PD (OR = 0.82, 95% CI: 0.44–1.51), although no significant difference (P = 0.470, Appendix S5). Three studies [37, 38, 40] reported the relationship between levels of bacteria in saliva samples and loss of attachment. One of them [40], showed that patients with high Lactobacillus (> 105 CFU/ml) in saliva had significantly higher average attachment loss (P = 0.014) and rate of sites with > 4 mm of attachment loss (P = 0.014). S. mutans followed the same trend as Lactobacillus. Similarly, another study by Robert et al. [37] showed that high levels of S. mutans (> 105 CFU/ml) in saliva were negatively correlated with a decrease in the sites of loss of attachment > 3 mm, OR adjusted = 0.74 (0.35–1.60), P = 0.447. Besides, this study reported a significant positive correlation between levels of F. nucleatum and S. mutans [OR adjusted = 6.03 (1.55–23.45), P = 0.009] as well as Actinobacillus [OR adjusted = 2.39 (1.00–5.71), P = 0.051]. What’s more, one study [38] reported the relationship of pathogenic bacteria in the saliva of patients with aggressive periodontitis, which found that the level of Actinobacillus in saliva was positively correlated with the proportion of deep periodontal pockets (> 7 mm), P = 0.03, and negatively correlated with the level of S. mutans. Among them, Actinobacillus was detectable in 25 out of 30 aggressive periodontitis (AgP) patients, 15 of which showed low levels of S. mutans in their saliva (< 103 CFU/ml), indicating a negative correlation (OR = 0.08, 95% CI: 0.01–0.85, P = 0.005) between them. Moreover, the level of Actinobacillus in saliva of patients with low level of S. mutans (< 103 CFU/ml) was significantly higher than that of patients with high level of S. mutans (> 105 CFU/ml) and medium level of S. mutans (103–105 CFU/ml), P = 0.009. Three studies [39, 41, 42] reported the effect of periodontal treatment on periodontitis and caries bacteria. As summarized in Appendix S6, compared to pre-treatment, the prevalence of high levels (> 104 CFU/ml) of S. mutans increased after scaling and root planning (OR = 1.54, 95% CI: 0.47–5.02), but there was no statistically significant difference. (P = 0.470). Nevertheless, the mean level of S. mutans was significantly higher (P < 0.05) after periodontal therapy. Another study [42] assessed levels of saliva and supragingival plaque during periodontal treatment. The amount of S. mutans in saliva increased significantly at 8th month (P < 0.05). For supragingival plaque, the CFUs of S. mutans remained basically unchanged, but the frequency of detection increased from 4/10 to 6/10. lactobacilli decreased significantly at 8th month compared to preoperative (P < 0.05).
Publication bias
Study publication bias was examined using funnel plot for caries risk in patients with PD in Appendix S7. Visual assessment of the Funnel plot revealed studies was displayed nearly symmetrical appearance, indicating no significant bias.
Discussion
This systematic review supported a positive association between PD and caries. In terms of caries condition in patients with PD, patients with moderate to severe periodontitis had a higher (57%) chance of having caries compared to non-periodontitis patients. In addition, a positive linear relationship was observed, confirming that the more severe the periodontitis was, the higher the likelihood of having caries. Based on the classification of caries between coronal and root caries, the study found that patients with periodontitis had an elevated likelihood of having root caries.
This systematic review also attempted for the first time to provide estimates of the mean DMFT and DFR in periodontitis patients. Interestingly, 50% of the included studies reported an increased DMFT in patients with periodontitis. However, all of the included studies showed an elevated mean DFR in patients with periodontitis. The association between periodontitis and root caries observed in this study was in agreement with previous studies [44], in which periodontitis was correlated with the prevalence of root caries.
In terms of the periodontal condition of patients with caries, patients also had a higher (79%) risk of having periodontitis compared to controls. This result was in line with the findings of the study [29], which showed that people with three or more untreated carious lesions were more likely to develop periodontal disease. The explanation for this phenomenon may be that untreated carious lesions may increase plaque retention and susceptibility to periodontal disease [45]. This was observed in a 3-year longitudinal study in which untreated carious lesions in adolescents had a significant negative impact on periodontal health [46].
The positive correlation between the two diseases can be explained by common risk factors, including oral hygiene practices [47], the presence of dental biofilms, lifestyle habits [48] and social factors [49]. Dental plaque biofilms, which are the initiators of caries and periodontitis, constituted distinct polymicrobial communities. With dynamic changes in community composition, microbial metabolism and by-products that triggered host immune responses can cause changes in the local environment, thereby affecting the growth or colonization of single or multiple microbial populations, promoting the growth or over-activity of microorganisms associated with ecological maladies, and thus influencing the development of the disease [17].
In the same oral environment, the positive correlation between caries and periodontitis was found to be closely related to its oral hygiene environment. Jiang et al. [50] analyzed the periodontal status of 86 pairs of carious teeth and found that the degree of gingival recession, the depth of periodontal probing, and calculus index were significantly higher in the carious group than in the no-caries group, regardless of whether they suffered only from crown caries or crown-root caries. This may be due to the fact that caries tends to occur on tooth surfaces that are not easily cleaned and where food debris tends to linger, and these areas are often where plaque tends to accumulate. As caries progressed, pain from food embedded in the cavity or temperature stimulation can occur, leading patients to unconsciously avoid these sensitive areas when brushing and rinsing, which in turn made plaque and calculus more likely to accumulate. This provided a favorable environment for the survival of periodontal pathogenic bacteria, leading to the development of periodontitis. Similarly, periodontitis caused the gums to recede, leading to root exposure. Due to the temperature sensitivity of the exposed roots, the teeth on the affected side were disused and prone to food debris accumulation. At the same time, the lack of periodontal tissue allowed for increased loss of teeth in severe periodontitis, resulting in larger gaps between the teeth, which made it easier for food to become embedded. The environment of the affected side favored the growth and metabolism of cariogenic bacteria to form caries. Therefore, the close relationship between oral hygiene, plaque biofilm and the two diseases can be used to support the positive correlation between caries and periodontitis.
In particular, one of the explanations for the strong association between periodontitis and root caries was that the exposure of cementum/dentine due to recession caused by PD may provide a new substrate for bacterial adhesion, and favor colonization of Gram-negative proteolytic species that can degrade the endogenous collagen and other organic components of these tissues [51]. Furthermore, bacteria metabolized sugar into organic acids, which initiated root surface demineralization by removing calcium and phosphate ions from surface apatite crystals. For enamel, this process took place as the pH reached the critical value of 5.5; however, pH 6.4 was sufficient for cementum and dentin demineralization, due to their lower degree of mineralization [52] as well as limited amount of fluoride and poorer caries resistance than enamel. Traditional periodontal treatment reducing the resistance to caries may be another reason that mechanical removal of plaque, scaling and root planning affected the outer part of the root surface and exposed cementum and dentin surfaces with low caries resistance [42]. In addition, gingival recession enables saliva to access the root surface, which is believed to be one of the most important host factors and an essential mediator controlling the speed and direction of the cariogenic pathway. Saliva was shown to neutralize the pH level on the root surface by a salivary buffering action and changed nutritional conditions, affecting the environment and nutrients of bacteria [13].
Apart from the correlation at the clinical level, this study also summarized the correlation between periodontitis and caries-related causative organisms. The results showed that patients with periodontitis had decreased salivary levels of S. mutans, one of the main caries-causing organisms, compared to those without periodontitis. Besides, several studies demonstrated that patients with high salivary levels of S. mutans (> 105 CFU/ml) had reduced mean attachment loss and the number of sites with attachment loss > 4 mm. These results could be explained by the fact that periodontitis mediates changes in the microecological community, leading to increased inflammation and alterations in the microbiological composition of oral biofilms [53]. Some of these reports suggested an antagonistic relationship between bacteria associated with the oral microbiota of both diseases. Numerous clinical trials have confirmed a negative correlation between P. gingivalis and S. mutans both in subgingival plaque and saliva before and after systemic treatment of periodontal disease patients [39]. Different degrees of antagonism were found between the two bacteria when they were co-cultured, in which P. gingivalis was found to interfere with the quorum sensing (QS) system of S. mutans, thereby diminishing its biofilm-forming ability, acid tolerance, and bacteriocin-producing ability [54]. However, some studies [37] have also shown a positive correlation between other periodontal pathogens such as F. nucleatum, Actinobacillus and S. mutans, which may be mediated by the co-aggregation of F. nucleatum [55] and the high genetic variability of Actinobacillus strain [56]. Therefore, further long-term cohort and elementary research on the bacterial association between periodontitis and caries should be carried out, in order to better understand the pathogenetic relationship between the two diseases, which can facilitate innovative strategies for the prevention and treatment of these two diseases with highly prevalence.
In this systematic review, for the first time, we conducted the correlation between PD and dental caries at the clinical and bacterial levels. Interestingly, either periodontitis or caries as independent variable, the correlation between the two diseases was positive and significant. In addition, the pooled results of all the included studies indicated that patients with periodontitis had a higher DMFT/DFR index. However, at the bacterial level, there seemed to be a negative correlation between the levels of the main dominant pathogenic bacteria related to two diseases in the same microecological environment. Studies have shown that environmental acidification was the main cause of phenotypic and genotypic changes in the microbiota during the development of dental caries [57], with S. mutans and Lactobacillus being identified as specific caries pathogens [11]. However, S. mutans was not only present at high levels in the early stages of dental caries, but also in healthy humans, even at very low levels [58, 59]. This seemingly contradictory results seemed to explain the trend related to the clinical and bacterial dimensions in this study, where both caries and periodontitis were based on a multiple microbiological community and a variety of other factors influencing the course of the disease, rather than being determined by a single or a select few bacterial species [4, 60]. In other words, dysbiosis contributed to diseases means an alteration in the abundance or influence of individual species within the polymicrobial community, relative to their abundance or influence in health. Whereas dysbiosis can lead to destructive periodontal inflammation, the reverse is also true. In this respect, inflammatory tissue breakdown products such as degraded collagen and heme-containing compounds are released into the gingival crevicular fluid. In the gingival crevice/pocket, these inflammatory spoils can be used as nutrients to fuel the selective expansion of a subset of bacterial species like proteolytic and asaccharolytic pathobionts, thereby exacerbating the imbalance of the dysbiosis [61,62,63]. In oral ecosystems, the proximity of microorganisms promoted a series of biochemical interactions that may be favorable to one organism and antagonistic to the other. Therefore, further studies combining clinical and microbiological measures of both these diseases are necessary for better understanding of the possible relationship between these two diseases which may lead to improved management and prevention.
Strengths and weaknesses
This systematic review was designed to comprehensively investigate the trends between caries and periodontitis. As a result, the bidirectional relationship between dental caries and periodontitis both in clinical and bacterial levels was first analyzed, and more precise conclusions through subgroup analysis were drawn. Meanwhile, this study analyzed the DMFT/DFR index of patients with periodontitis for the first time, and especially the strong correlation with root caries was important for the treatment and prevention of patients with periodontitis. For example, the inclusion of caries prevention treatment in periodontal sequence therapy or the adjustment of the follow-up time for patients with severe periodontitis was important in PD therapy. Furthermore, the correlation between two diseases at the bacterial level suggested that symbiosis or antagonism between one or several pathogenic bacteria may not directly determine the progression of the disease, but may also cause the disease through affecting the oral microecological balance, bacterial quorum sensing (QS) mechanism and host-community interaction. Therefore, further research in identifying the interplay between pathogens in each individual and their relative contribution on each other is needed. However, a number of limitations should be highlighted starting with the limited value of this systematic reviews of observational studies for ascertaining causality. Moreover, the definition of periodontitis in several studies seemed a slight discrepancy as well as bias in clinical examination, which may lead to selection bias. Not all included studies were adjusted for potential confounders such as diet, smoking habit and socio-economic level. The true value could be distorted by these confounding factors. Further intervention studies and long-term are needed to establish this causal relationship. What’s more, Due to the limited number of included studies and subjects, no further subgroup analysis between sexes or ages were performed.
Conclusions
This study demonstrated that both of two disorders were linked by two-way relationships. Especially, the risk of dental caries was increased by the severity of periodontitis. The presence of periodontitis was also associated with an increased DMFT and DFR. At the bacterial level, however, the pathogens associated with two disorders were negatively correlated. Our findings highlighted the necessity to improve caries prevention during periodontal treatment. Longer and larger studies are needed however to determine whether periodontal treatment with concomitant caries management facilitates disease therapy and prognosis, and how intrinsic bacterial interactions or immune regulation affect the disease, ultimately resulting in reduced morbidity.
Data Availability
The authors confirmed that the data supporting the findings of this study were available in Table 1 of this manuscript and supplementary materials.
References
Buset SL, Walter C, Friedmann A et al (2016) Are periodontal diseases really silent? A systematic review of their effect on quality of life [J]. J Clin Periodontol 43(4):333–344
Frencken JE, Sharma P, Stenhouse L et al (2017) Global epidemiology of dental caries and severe periodontitis - a comprehensive review [J]. J Clin Periodontol 44 Suppl 18:S94–S105
Kassebaum NJ, Bernabé E, Dahiya M et al (2014) Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression [J]. J Dent Res 93(11):1045–53
Van Dyke TE, Bartold PM, Reynolds EC (2020) The Nexus Between Periodontal Inflammation and Dysbiosis [J]. Front Immunol 11:511
Lamont RJ, Koo H, Hajishengallis G (2018) The oral microbiota: dynamic communities and host interactions [J]. Nat Rev Microbiol 16(12):745–759
Bartold PM (2018) Lifestyle and periodontitis: The emergence of personalized periodontics [J]. Periodontol 2000 78(1):7–11
Winning L, Linden GJ (2015) Periodontitis and systemic disease [J]. BDJ Team 2(10):7–10
Preshaw PM, Alba AL, Herrera D et al (2012) Periodontitis and diabetes: a two-way relationship [J]. Diabetologia 55(1):21–31
Koziel J, Potempa J (2022) Pros and cons of causative association between periodontitis and rheumatoid arthritis [J]. Periodontol 2000 89(1):83–98
Leira Y, Domínguez C, Seoane J et al (2017) Is Periodontal Disease Associated with Alzheimer’s Disease? A Systematic Review with Meta-Analysis [J]. Neuroepidemiology 48(1–2):21–31
Selwitz RH, Ismail AI, Pitts NB (2007) Dental caries [J]. Lancet 369(9555):51–59
Jain N, Dutt U, Radenkov I et al (2023) WHO’s global oral health status report 2022: Actions, discussion and implementation [J]. Oral Dis 30:73–79
Gao X, Jiang S, Koh D et al (2016) Salivary biomarkers for dental caries [J]. Periodontol 2000 70(1):128–41
Fejerskov O (2004) Changing paradigms in concepts on dental caries: consequences for oral health care [J]. Caries Res 38(3):182–191
Kidd EA, Fejerskov O (2004) What constitutes dental caries? Histopathology of carious enamel and dentin related to the action of cariogenic biofilms [J]. J Dent Res 83 Spec No C:C35-8
Cheng L, Zhang L, Yue L et al (2022) Expert consensus on dental caries management [J]. Int J Oral Sci 14(1):17
Hajishengallis G (2015) Periodontitis: from microbial immune subversion to systemic inflammation [J]. Nat Rev Immunol 15(1):30–44
Curtis MA, Zenobia C, Darveau RP (2011) The relationship of the oral microbiotia to periodontal health and disease [J]. Cell Host Microbe 10(4):302–306
Darveau RP (2010) Periodontitis: a polymicrobial disruption of host homeostasis [J]. Nat Rev Microbiol 8(7):481–490
Holt SC, Ebersole JL (2005) Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis [J]. Periodontol 2000 38:72–122
Fine DH, Goldberg D, Karol R (1984) Caries levels in patients with juvenile periodontitis [J]. J Periodontol 55(4):242–246
Sewón LA, Parvinen TH, Sinisalo TV et al (1988) Dental status of adults with and without periodontitis [J]. J Periodontol 59(9):595–8
Page RC, Eke PI (2007) Case definitions for use in population-based surveillance of periodontitis [J]. J Periodontol 78(7 Suppl):1387–1399
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses [J]. Eur J Epidemiol 25(9):603–605
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis [J]. Stat Med 21(11):1539–1558
Martínez M, Montero E, Carasol M et al (2021) Association between caries and periodontal diseases in a sample of employed adults in Spain: a cross-sectional study [J]. Clin Oral Investig 25(6):3957–66
Vehkalahti M, Paunio I (1994) Association between root caries occurrence and periodontal state [J]. Caries Res 28(4):301–306
Naimi-Akbar A, Kjellström B, Rydén L et al (2019) Attitudes and lifestyle factors in relation to oral health and dental care in Sweden: a cross-sectional study [J]. Acta Odontol Scand 77(4):282–9
Strauss FJ, Espinoza I, Stähli A et al (2019) Dental caries is associated with severe periodontitis in Chilean adults: a cross-sectional study [J]. BMC Oral Health 19(1):278
Belstrøm D, Fiehn NE, Nielsen CH et al (2014) Differences in bacterial saliva profile between periodontitis patients and a control cohort [J]. J Clin Periodontol 41(2):104–12
Tefera AT, Girma B, Adane A et al (2022) Periodontal status of students living with disability in Amhara region, Ethiopia: a cross-sectional study [J]. BMC Oral Health 22(1):343
Mattila PT, Niskanen MC, Vehkalahti MM et al (2010) Prevalence and simultaneous occurrence of periodontitis and dental caries [J]. J Clin Periodontol 37(11):962–967
Fadel H, Al Hamdan K, Rhbeini Y et al (2011) Root caries and risk profiles using the Cariogram in different periodontal disease severity groups [J]. Acta Odontol Scand 69(2):118–24
Alqobaly L, Sabbah W (2020) The association between periodontal disease and root/coronal caries [J]. Int J Dent Hyg 18(1):99–106
Yu LX, Wang X, Feng XP et al (2021) The relationship between different types of caries and periodontal disease severity in middle-aged and elderly people: findings from the 4th National Oral Health Survey of China [J]. BMC Oral Health 21(1):229
Yildiz Telatar G, Gürlek B, Telatar BC (2021) Periodontal and caries status in unexplained female infertility: A case-control study [J]. J Periodontol 92(3):446–54
Durand R, Roufegarinejad A, Chandad F et al (2019) Dental caries are positively associated with periodontal disease severity [J]. Clin Oral Investig 23(10):3811–3819
Kozlovsky A, Wolff A, Saminsky M et al (2015) Effect of Aggregatibacter actinomycetemcomitans from Aggressive Periodontitis patients on Streptococcus mutans [J]. Oral Dis 21(8):955–961
Van Der Reijden WA, Dellemijn-Kippuw N, Stijne-Van Nes AM et al (2001) Mutans streptococci in subgingival plaque of treated and untreated patients with periodontitis [J]. J Clin Periodontol 28(7):686–91
Saotome Y, Tada A, Hanada N et al (2006) Relationship of cariogenic bacteria levels with periodontal status and root surface caries in elderly Japanese [J]. Gerodontology 23(4):219–225
Iwano Y, Sugano N, Matsumoto K et al (2010) Salivary microbial levels in relation to periodontal status and caries development [J]. J Periodontal Res 45(2):165–169
Quirynen M, Gizani S, Mongardini C et al (1999) The effect of periodontal therapy on the number of cariogenic bacteria in different intra-oral niches [J]. J Clin Periodontol 26(5):322–327
Shrestha S, Shrestha RM (2016) Correlation Between Oral Health and Body Mass Index among Nepalese Teachers [J]. Kathmandu Univ Med J (KUMJ) 14(55):231–234
López R, Smith PC, Göstemeyer G et al (2017) Ageing, dental caries and periodontal diseases [J]. J Clin Periodontol 44 Suppl 18:S145–S52
Nascimento GG, Baelum V, Dahlen G et al (2018) Methodological issues in assessing the association between periodontitis and caries among adolescents [J]. Commun Dent Oral Epidemiol 46(3):303–309
Albandar JM, Buischi YA, Axelsson P (1995) Caries lesions and dental restorations as predisposing factors in the progression of periodontal diseases in adolescents. A 3-year longitudinal study [J]. J Periodontol 66(4):249–54
Jepsen S, Blanco J, Buchalla W et al (2017) Prevention and control of dental caries and periodontal diseases at individual and population level: consensus report of group 3 of joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases [J]. J Clin Periodontol 44 Suppl 18:S85–S93
Sakki TK, Knuuttila ML, Vimpari SS et al (1995) Association of lifestyle with periodontal health [J]. Commun Dent Oral Epidemiol 23(3):155–158
Sälzer S, Alkilzy M, Slot DE et al (2017) Socio-behavioural aspects in the prevention and control of dental caries and periodontal diseases at an individual and population level [J]. J Clin Periodontol 44 Suppl 18:S106–S15
江千舟,王丹,缪晓琳,等.龋病患牙牙周健康状况调查分析[J].口腔医学研究, 2009, 25(4):3.DOI:CNKI:SUN:KQYZ.0.2009–04–043
Gavriilidou NN, Belibasakis GN (2019) Root caries: the intersection between periodontal disease and dental caries in the course of ageing [J]. Br Dent J 227(12):1063–1067
Melberg JR (1986) Demineralization and remineralization of root surface caries [J]. Gerodontology 5(1):25–31
Freire M, Nelson KE, Edlund A (2021) The Oral Host-Microbial Interactome: An Ecological Chronometer of Health? [J]. Trends Microbiol 29(6):551–561
Li YH, Tian X (2012) Quorum sensing and bacterial social interactions in biofilms [J]. Sensors (Basel) 12(3):2519–2538
Guo L, Shokeen B, He X et al (2017) Streptococcus mutans SpaP binds to RadD of Fusobacterium nucleatum ssp. polymorphum [J]. Mol Oral Microbiol 32(5):355–64
Kaplan JB, Schreiner HC, Furgang D et al (2002) Population structure and genetic diversity of Actinobacillus actinomycetemcomitans strains isolated from localized juvenile periodontitis patients [J]. J Clin Microbiol 40(4):1181–1187
Takahashi N, Nyvad B (2011) The role of bacteria in the caries process: ecological perspectives [J]. J Dent Res 90(3):294–303
Gross EL, Leys EJ, Gasparovich SR et al (2010) Bacterial 16S sequence analysis of severe caries in young permanent teeth [J]. J Clin Microbiol 48(11):4121–4128
Requena T, Velasco M (2021) The human microbiome in sickness and in health [J]. Rev Clin Esp (Barc) 221(4):233–240
Kornman KS (2008) Mapping the pathogenesis of periodontitis: a new look [J]. J Periodontol 79(8 Suppl):1560–1568
Rosier BT, Marsh PD, Mira A (2018) Resilience of the Oral Microbiota in Health: Mechanisms That Prevent Dysbiosis [J]. J Dent Res 97(4):371–380
Hajishengallis G (2014) The inflammophilic character of the periodontitis-associated microbiota [J]. Mol Oral Microbiol 29(6):248–257
Herrero ER, Fernandes S, Verspecht T et al (2018) Dysbiotic Biofilms Deregulate the Periodontal Inflammatory Response [J]. J Dent Res 97(5):547–555
Funding
This work was supported by the Science and Technology Innovation Project for postgraduate of Hubei University of Medicine (No. YC2023055), the Scientific Research funding Project of Hubei Provincial Department of Education (No. Q20222114), the Scientific Research guiding Project of Hubei Provincial Department of Education (No. B2022499) and the Scientific Research Project of Shiyan Taihe hospital (No. 2023JJXM038).
Author information
Authors and Affiliations
Contributions
Y.Li, Y.Xiang and H.Ren contributed to methodology, data analysis and interpretation, and to manuscript drafting. C.Zhang and Z.Hu contributed to conceptualization, Investigation, Software and reviewed the manuscript. W.Leng and L.Xia contributed to study conception and design, data analysis and interpretation, and critically revised the manuscript. All the authors gave their final approval of the version to be published and agreed to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, author- ship, and publication of this article.
Ethics Approval and Consent to Participate
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Xiang, Y., Ren, H. et al. Association between periodontitis and dental caries: a systematic review and meta-analysis. Clin Oral Invest 28, 306 (2024). https://doi.org/10.1007/s00784-024-05687-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-024-05687-2