Abstract
Objectives
This study aimed to compare the antimicrobial action, cytotoxicity, cleaning ability, and erosion of dentine of hypochlorous acid (HClO) obtained from an electrolytic device at two different concentrations (Dentaqua) and three concentrations of sodium hypochlorite (NaOCl).
Methods
Microbiological test—The root canals of sixty single-rooted extracted human teeth were inoculated with Enterococcus faecalis and divided into 6 groups (n = 10), according to decontamination protocol: DW (control); 1% NaOCl; 2.5% NaOCl; 5.25% NaOCl; 250 ppm HClO and 500 ppm HClO. The colony-forming units were counted to evaluate the decontamination potential of each group, calculating the reduction in bacterial percentage. Cytotoxicity test—Cytotoxicity was evaluated after inoculation of the same tested protocols in fibroblastic cells for 3 min, calculating the cell viability percentages. Specifical statistical analysis was performed (α = 5%). Cleaning ability and erosion—Fifty-six single-rooted bovine lower incisors were divided into seven groups of 8 roots each, being the test groups 1% NaOCl; 2.5% NaOCl; 5,25% NaOCl; 250 ppm HClO and 500 ppm HClO, and a negative and positive control. Negative control was not contaminated, and the other groups were inoculated with Enterococcus faecalis. SEM images were ranked as from the cleanest to the least clean. Erosion was also assessed, being ranked from the least to the most eroded dentine.
Results
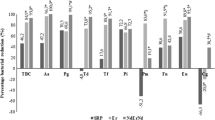
The highest bacterial reduction was observed in experimental groups, with no statistical differences between them (p > 0.05). The highest number of viable cells was observed in control group, followed by 250 ppm HClO and 500 ppm HClO groups, with statistical differences between them (p < 0.05). 1% NaOCl; 2.5% NaOCl; 5.25% NaOCl and 500 ppm HClO displayed the cleanest areas. All sodium hypochlorite groups displayed erosion with higher ranks with greater concentration, while hypochlorous acid did not display any erosion regardless the concentration.
Conclusions
It is possible to conclude that HClO obtained from an electrolytic device presented high antimicrobial activity and low cytotoxicity in both tested concentrations. 500 ppm HClO did not display erosion and showed great cleaning ability.
Clinical relevance
The use of 500 ppm hypochlorous acid may reduce unfavorable behavior of sodium hypochlorite whilst maintaining its antimicrobial action.






Similar content being viewed by others
References
Kakehashi S, Stanley HR, Fitzgerald RJ (1965) The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol 20:340–349
Pai ARV (2023) Injection of sodium hypochlorite into soft tissues of the oral cavity: A literature review with clinical preventive recommendations. J Stomatol Oral Maxillofac Surg 124:101581
Ahmed HM, Versiani MA, De-Deus G, Dummer PM (2017) A new system for classifying root and root canal morphology. Int Endod J 50:761–770
Siqueira Junior JF, Rôças IDN, Marceliano-Alves MF, Pérez AR, Ricucci D (2018) Unprepared root canal surface areas: causes, clinical implications, and therapeutic strategies. Braz Oral Res 32(suppl 1):e65
Du T, Ma J, Yang P, Xiong Z, Lu X, Cao Y (2012) Evaluation of antibacterial effects by atmospheric pressure nonequilibrium plasmas against Enterococcus faecalis biofilms in vitro. J Endod 38:545–549
Okino LA, Siqueira EL, Santos M, Bombana AC, Figueiredo JA (2004) Dissolution of pulp tissue by aqueous solution of chlorhexidine digluconate and chlorhexidine digluconate gel. Int Endod J 37:38–41
Moreira DM, Almeida JF, Ferraz CC, Gomes BP, Line SR, Zaia AA (2009) Structural analysis of bovine root dentin after use of different endodontics auxiliary chemical substances. J Endod 35:1023–1027
Tirali RE, Turan Y, Akal N, Karahan ZC (2009) In vitro antimicrobial activity of several concentrations of NaOCl and Octenisept in elimination of endodontic pathogens. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108:117–120
Abou-Rass M, Oglesby SW (1981) The effects of temperature, concentration, and tissue type on the solvent ability of sodium hypochlorite. J Endod 7:376–377
Alkahtani A, Alkahtany SM, Anil S (2014) An in vitro evaluation of the cytotoxicity of varying concentrations of sodium hypochlorite on human mesenchymal stem cells. J Contemp Dent Pract 15:473–481
Xu H, Ye Z, Zhang A, Lin F, Fu J, Fok ASL (2022) Effects of concentration of sodium hypochlorite as an endodontic irrigant on the mechanical and structural properties of root dentine: A laboratory study. Int Endod J 55:1091–1102
Zehnder M (2006) Root canal irrigants. J Endod 32:389–398
Retana-Lobo C, Ramírez-Mora T, Murillo-Gómez F, Maria Guerreiro-Tanomaru J, Tanomaru-Filho M, Reyes-Carmona J (2022) Final irrigation protocols affect radicular dentin DMP1-CT expression, microhardness, and biochemical composition. Clin Oral Investig 26:5491–5501
Renovato SR, Oliveira MM, Siqueira PC, Silva JA, Decurcio DA (2017) Análise da erosão da dentina radicular após irrigação com hipoclorito de sódio em diferentes concentrações por meio de microscopia eletrônica de varredura. ROBRAC 26(79):26–31
Kawanishi Y, Maezono H, Shimaoka T, Kitatani T, Naito K, Sotozono M, Klanliang K, Takahashi Y, Hayashi M (2023) Morphological analyses of effects of endodontic irrigant solutions using a root canal model and an immersion model. Int J Dent 2023:3938522
Martin MV, Gallagher MA (2005) An investigation of the efficacy of super-oxidised water (Optident/Sterilox) water for the disinfection of dental unit waterlines. Br Dent J 198:353–354
Boyle MA, O’Donnell MJ, Russell RJ, Coleman DC (2010) Lack of cytotoxicity by Trustwater Ecasol™ used to maintain good quality dental unit waterline output water in keratinocyte monolayer and reconstituted human oral epithelial tissue models. J Dent 38:930–940
Sam CH, Lu HK (2009) The role of hypochlorous acid as one of the reactive oxygen species in periodontal disease. J Dent Sci 4:45–54
Vijayaraghavan K, Ramanujam TK, Balasubramanian N (1999) In situ hypochlorous acid generation for the treatment of distillery spentwash. Ind Eng Chem Res 38:2264–2267
Tsai CF, Chung JJ, Ding SJ, Chen CC (2024) In vitro cytotoxicity and antibacterial activity of hypochlorous acid antimicrobial agent. J Dent Sci 19(1):345–356
Gründling GL, Zechin JG, Jardim WM, de Oliveira SD, de Figueiredo JA (2011) Effect of ultrasonics on Enterococcus faecalis biofilm in a bovine tooth model. J Endod 37:1128–1133
Kim SG, Kahler B, Lin LM (2016) Current developments in regenerative endodontics. Curr Oral Health Rep 3:293–301
Sedgley CM, Lennan SL, Appelbe OK (2005) Survival of Enterococcus faecalis in root canals ex vivo. Int Endod J 38:735–742
Guerreiro-Tanomaru JM, de Faria-Júnior NB, Duarte MA, Ordinola-Zapata R, Graeff MS, Tanomaru-Filho M (2013) Comparative analysis of Enterococcus faecalis biofilm formation on different substrates. J Endod 39:346–350
Evans MD, Baumgartner JC, Khemaleelakul S, Xia T (2003) Efficacy of calcium hydroxide: chlorhexidine paste as an intracanal medication in bovine dentin. J Endod 29:338–339
Menezes MM, Valera MC, Jorge AO, Koga-Ito CY, Camargo CH, Mancini MN (2004) In vitro evaluation of the effectiveness of irrigants and intracanal medicaments on microorganisms within root canals. Int Endod J 37:311–319
Eldeniz AU, Mustafa K, Ørstavik D, Dahl JE (2007) Cytotoxicity of new resin-, calcium hydroxide- and silicone-based root canal sealers on fibroblasts derived from human gingiva and L929 cell lines. Int Endod J 40:329–337
Barnhart BD, Chuang A, Dalle Lucca JJ, Roberts S, Liewehr F, Joyce AP (2005) An in vitro evaluation of the cytotoxicity of various endodontic irrigants on human gingival fibroblasts. J Endod 31:613–615
Souza MA, Menon CZ, Nery LF, Bertol CD, Rossato-Grando LG, Cecchin D (2018) Effect of root canal preparation techniques on chlorhexidine substantivity on human dentin: a chemical analysis. Clin Oral Investig 22:859–865
Souza MA, Lago BLT, Pletsch A, Binotto A, Poletti A, Rodrigues FT, Ricci R, Bischoff KF, Dias CT, Palhano HS, Lago CTR, Farina AP, Cecchin D, Bervian J, de Figueiredo JAP (2020) Association of calcium hypochlorite, reciprocating instrumentation and photodynamic therapy: Antimicrobial analysis and effects on root dentin structure. Photodiagnosis Photodyn Ther 29:101625
Siqueira JF Jr, Rôças IN, Paiva SS, Guimarães-Pinto T, Magalhães KM, Lima KC (2007) Bacteriologic investigation of the effects of sodium hypochlorite and chlorhexidine during the endodontic treatment of teeth with apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 104:122–130
Siqueira JF Jr, Rôças IN (2008) Clinical implications and microbiology of bacterial persistence after treatment procedures. J Endod 34:1291–1301
Baumgartner JC, Ibay AC (1987) The chemical reactions of irrigants used for root canal debridment. J Endod 13:47–51
del Carpio-Perochena A, Bramante CM, de Andrade FB, Maliza AG, Cavenago BC, Marciano MA, Amoroso-Silva P, Duarte MH (2015) Antibacterial and dissolution ability of sodium hypochlorite in different pHs on multi-species biofilms. Clin Oral Investig 19:2067–2073
Gandolfi MG, Taddei P, Pondrelli A, Zamparini F, Prati C, Spagnuolo G (2018) Demineralization, Collagen Modification and Remineralization Degree of Human Dentin after EDTA and Citric Acid Treatments. Materials (Basel) 12(1):25
Wagner MH, da Rosa RA, de Figueiredo JAP, Duarte MAH, Pereira JR, Só MVR (2017) Final irrigation protocols may affect intraradicular dentin ultrastructure. Clin Oral Investig 21(7):2173–2182
Pace R, Di Nasso L, Tauro L, Nizzardo A, Pagavino G, Giuliani V (2020) Analysis of dentinal erosion and removing smear layer of different irrigation protocols: an in vitro study. Giornale Italiano Endodonzia 34:13–21
Castagnola R, Martini C, Colangeli M, Pellicciotta I, Marigo L, Grande NM, Bugli F, Plotino G (2024) In vitro evaluation of smear layer and debris removal and antimicrobial activity of different irrigating solutions. Eur Endod J 9:81–88
Funding
This work has no financial support.
Author information
Authors and Affiliations
Contributions
Matheus Albino Souza—supervising all experimental tests and writing the article.
Gabriele Vanin—performing microbiological and cytotoxicity tests.
Mylena Zanella—performing microbiological and cytotoxicity tests.
Camila Pizzi—performing microbiological and cytotoxicity tests.
Eduarda Ferreira—performing microbiological and cytotoxicity tests.
Felipe Dallepiane—performing microbiological and cytotoxicity tests.
Nathan Piccolo—performing microbiological and cytotoxicity tests.
Jordana da Silva Koch – performing SEM cleaning and erosion tests.
Kellyn Rocca Souza – performing SEM cleaning and erosion tests.
Aleksandra Palatynska-Ulatowska—performing SEM cleaning and erosion tests.
Ubirajara Maciel da Costa – supervising the cytotoxicity test.
Vanessa Valgas dos Santos – supervising the cytotoxicity test.
Liviu Steier – supervising all experimental tests and writing the article.
José Antonio Poli de Figueiredo—supervising all experimental tests and writing the article.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Research Ethical Committee of the University of Passo Fundo (protocol No. 5.783.928).
Informed consent
Informed consent is not applicable because samples were composed of extracted human teeth that were obtained from the Biobank.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Souza, M.A., Steier, L., Vanin, G.N. et al. Antimicrobial action, cytotoxicity and erosive potential of hypochlorous acid obtained from an electrolytic device compared with sodium hypochlorite. Clin Oral Invest 28, 282 (2024). https://doi.org/10.1007/s00784-024-05675-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-024-05675-6