Abstract
Objectives
To evaluate the risk and intensity of tooth sensitivity (TS), and the efficacy of in-office bleaching after applying an experimental desensitizing gel composed of 10% calcium gluconate, 0.1% dexamethasone acetate, 10% potassium nitrate, and 5% glutaraldehyde.
Material and methods
In a split-mouth, double-blind, placebo-controlled study, 50 participants had their upper hemiarches randomized into experimental and placebo groups. Desensitizing and placebo gels were applied for 10 min before in-office bleaching (35% hydrogen peroxide, 1 × 50 min; two bleaching sessions; 1-week interval). TS was recorded immediately after bleaching, 1, 24, and 48 h after each session, with a 0–10 visual analogue scale (VAS) and a five-point numerical rating scale (NRS). The color was recorded in all groups at baseline, 1 week after each session, and 1 month after the end of bleaching using shade guide units (ΔSGUs) and a spectrophotometer (ΔEab, ΔE00, and ΔWID).
Results
Most participants (96%) felt some discomfort during treatment regardless of the study group. The odds ratio for pain was 0.65 (95% CI 0.1 to 4.1; p = 1.0). The intensity of TS did not differ between groups (p > 0.31), and it was only 0.34 VAS units lower in the experimental group. A significant color change occurred in both groups regardless of the group.
Conclusions
The desensitizing experimental gel applied before in-office bleaching did not reduce the risk and the intensity of TS and did not affect color change.
Clinical relevance
Although the experimental desensitizing agent with varying mechanisms of action did not jeopardize the color change, it did not reduce the risk or intensity of in-office bleaching.
Clinical trial registration number
RBR-7T7D4D.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Some studies have shown that a large portion of the population is dissatisfied with the color of their teeth [1, 2]. This explains why clinicians have widely recommended and patients have widely accepted dental bleaching, either via the at-home or the in-office protocol, for obtaining esthetically pleasing smiles [3, 4].
Unlike at-home bleaching, in-office bleaching requires the use of high concentrations of hydrogen peroxide (HP) [5, 6]. However, the same HP that whitens teeth by oxidizing the dental structure’s organic component can also quickly diffuse into the pulp chamber [7]. This can trigger an inflammatory process [8] with the release of several inflammatory chemical mediators [9, 10]. This process modifies the local microcirculation, generating pressure over the peripheral nerve fibers and activating nociceptors [11]. Most patients experience bleaching-induced tooth sensitivity (TS) as a clinical consequence. This pain is characterized as acute and transient pain, with patients commonly reporting it within the first 24 h after in-office dental bleaching [12].
The preventive effect of analgesics and opioids [13, 14], anti-inflammatories [15,16,17,18], antioxidants [19], and corticosteroids [20,21,22] has previously been investigated, and they could not mitigate bleaching-induced TS [23]. So far, the most successful approach to reducing TS has been the topical application of desensitizers, such as those containing glutaraldehyde [24, 25], potassium nitrate [26, 27], and calcium agents [28, 29].
These topical agents’ mechanisms of action are different. Potassium nitrate prevents the repolarization of nerve fibers blocking the transmission of painful stimuli [26, 27, 30]. Meanwhile, glutaraldehyde was reported to coagulate proteins from enamel and dentinal tubules, reducing the easy passage of HP to the pulp [25, 31, 32]. Calcium-containing agents can also reduce the risk and intensity of TS mainly through the saturation of components on the enamel surface [33]. When calcium-containing products are applied, they interact with the dental surface [33]. They can be retained on the teeth, thus providing large amounts of calcium and phosphates for tissue interaction, which may reduce the passage of HP to the pulp [28, 29, 33,34,35,36]. Another possible agent is dexamethasone; this drug has already been tested orally [20, 22] but has not yet been investigated in topical form. This drug has primarily been used in dentistry via the oral route for oral surgeries [37,38,39] and endodontic treatments [40, 41], due to its potent anti-inflammatory effects. Although oral use of dexamethasone was not effective in reducing TS after dental bleaching [20, 22], its relatively smaller molar mass (392 g/mol−1) suggests that it can penetrate enamel and dentin, which may justify its topical use. In addition, dexamethasone is known as inhibit the expression of several inflammatory mediators and cytokines [42], which could promote an anti-inflammatory and analgesic effect by its possible contact with the dental pulp, in an attempt to reduce TS in the present study.
The mechanism of bleaching-induced TS is not yet entirely known. We hypothesized that summing up some active agents’ varying mechanisms of action could produce a more potent desensitizing effect than their individual use could. Therefore, we aimed to evaluate the impact of the topical application of this experimental desensitizing gel on the absolute risk and intensity of bleaching-induced TS and color change after in-office bleaching with 35% HP.
Material and methods
Ethics approval and protocol registration
This clinical investigation received approval (protocol 3.893.891) from the Ethics Committee of the State University of Ponta Grossa/PR/Brazil. This study was registered in the Brazilian Clinical Trials Registry under “RBR-7T7D4D.” The preparation of this article followed the protocol established via the Consolidated Standards of Reporting Trials statement with extension for within-person designs [43].
Trial design, settings, and location of data collection
This study was a randomized, split-mouth, placebo-controlled, and double-blind controlled clinical trial. This study was performed from November 2019 to January 2020 in the clinics of the school of dentistry at the State University of Ponta Grossa/PR/Brazil.
Recruitment
Recruitment was performed by placing written advertisements on the university walls and using social media to obtain a convenient sample. The volunteers were informed about the study’s objectives, and they all signed an informed consent form before being enrolled in the study.
Eligibility criteria
Participants included in this RCT were at least 18 years old, had good general and oral health, and did not report any type of TS. The participants were required to have six caries-free maxillary anterior teeth without restorations and periodontal disease. The canines had to be shade A2 or darker as judged by comparison with a value-oriented shade guide (Vita Classical, Vita Zahnfabrik). Participants with anterior restorations, dental prostheses, orthodontic apparatuses, and severe internal tooth discoloration (tetracycline stains, fluorosis, and pulpless teeth) were not included. In addition, pregnant or lactating women, smokers, participants who had bruxism and had undergone tooth-bleaching procedures, and any other condition that could cause sensitivity (such as recession, dentin exposure, or the presence of visible cracks in the teeth), and participants with continuous use of anti-inflammatory drugs or analgesics were also excluded.
Sample size estimation
This study’s primary outcome was the absolute risk of TS. The absolute risk of TS was reported to be approximately 93% for the bleaching product Whiteness Automixx (FGM) [44]. For detecting an absolute risk difference of 25% between the control and experimental groups, a minimum sample size of 40 patients with a power of 80% and an alpha of 5% was required. Due to the high cost of the search for study participants during the follow-ups, we included 50 participants.
Randomization
We performed blocked randomization (block size of 2) to guarantee equal–sized groups with an equal allocation ratio at www.sealedenvelope.com. A third party not involved in the study implementation prepared consecutively numbered, opaque, and sealed envelopes containing information identifying the groups. The group identified in the envelope corresponded to the treatment performed on the right upper hemiarch, and the left hemiarch received the alternate treatment.
Blinding
This study was a double-blind study in which the patients and evaluators were blinded to the group assignment. As the gels differed slightly in the transparency, we could not blind the operator. The groups (experimental or placebo) were applied in the superior and inferior arches before the in-office dental bleaching.
Study intervention
We prepared an experimental desensitizing gel containing 10% calcium gluconate, 0.1% dexamethasone acetate, 10% potassium nitrate, and 5% glutaraldehyde. Also, it was used an excipient to prepare the experimental desensitizing (100 g). We used propylene glycol and hydroxyethylcellulose as thickening agents, and we used methylparaben as a preservative. The placebo gel was composed of the same thickening agents and preservative without the active components to maintain the same viscosity and appearance. Although all attempts were made to produce a placebo gel similar to the experimental gel, the final product showed a different transparency.
All participants underwent dental prophylaxis and oral hygiene guidance prior to the bleaching procedure. After a lip retractor (Arcfex, FGM, Joinville, SC, Brazil) was placed in the proper position, the gingival tissue was isolated with a light-cured resin dam (Topdam, FGM, Joinville, SC, Brazil). An extension of the barrier was created between the central incisors so that the products would not contact each other.
Before each bleaching session, the experimental or placebo gel was applied topically on the buccal surfaces of all of the teeth to be bleached. The gel was left undisturbed for 10 min and then activated for 10 s with a micro brush. The application of the gels was carried out in the upper and lower arches. After the application, the gels were removed with gauze and were washed with an air–water spray.
The 35% HP bleaching gel (Whiteness HP Automixx, FGM, Joinville, SC, Brazil) was applied in a 50-min session. At the end of the recommended time, the bleaching gel was removed with a disposable surgical saliva ejector, cleaned with gauze, and washed with an air–water spray. Two bleaching sessions were performed at a 1-week interval.
Evaluation of tooth sensitivity (TS)
Participants had to record their pain intensity in the following time intervals: (1) during the treatment; (2) up to 1 h after each bleaching session; (3) between 1 and 24 h after each bleaching session; and (4) between 24 and 48 h. After both bleaching sessions, these measurements were performed using the five-point numerical rating scale (NRS; 0 = none, 1 = mild, 2 = moderate, 3 = considerable, and 4 = severe) [21, 26, 44, 45] and 0–10 visual analog scale (VAS) [21, 26, 44, 45]. The VAS scale is a 10-cm horizontal line with scores of zero and 10 at their ends, in which zero means no sensitivity and 10 means severe tooth sensitivity. The patient had to mark with a vertical line across the horizontal line of the scale the intensity of the TS. Then, the distance in millimeters from the zero end was measured with the aid of a millimeter ruler.
The worst score (NRS) or numerical value (VAS) obtained from all-time recalls were considered for statistical purposes. A patient who was insensitive to bleaching needed to score zero (no TS) during all assessments from both bleaching sessions. Participants were supposed to have TS to the bleaching procedure in all other circumstances. This dichotomization made it possible to calculate the absolute risk of TS, which represented the percentage of participants who reported TS at least once during treatment.
Color change
Two calibrated operators performed color evaluation before the bleaching session, 1 week after the first bleaching session, 1 week after the second treatment, and 1 month after the bleaching treatment. The final color measurement was planned to be collected 30 days after bleaching. However, because the end of the present study was coincident with the rise of the COVID-19 pandemic, 23 patients had their final color changes evaluated within 2 to 6 months. The color evaluation was never performed immediately after each bleaching session so that the effect of dehydration and demineralization on color measures could be avoided. The color evaluation was performed with the value-oriented shade guide Vita Classical (Vita Zahnfabrik) and the Vita Bleachedguide 3D-MASTER (Vita Zahnfabrik). In addition, an objective color evaluation was performed with the spectrophotometer Vita Easyshade (Vita Zahnfabrik).
The 16 shade guide tabs from the Vita Classical shade guide were arranged from the highest (B1) to the lowest (C4) value for the subjective examination. Although this scale is not linear in the truest sense, we treated the changes as representing a continuous and approximately linear ranking for analysis as already performed in published studies [21, 25, 27, 44, 45]. The Vita Bleachedguide 3D-MASTER contains lighter shade tabs and is organized from the highest (0M1) to the lowest (5M3) value.
The middle third of the right upper canine was used as the tooth-matching area. Color changes were calculated from the beginning of the active phase up to the individual recall times by calculating the difference in the number of shade guide units (∆SGUs), which occurred toward the lighter end of the value-oriented list of shade tabs. In the event of disagreement between the operators, the operators had to reach a consensus before the patient was dismissed.
For the objective evaluation, a preliminary impression of the maxillary arch was made with high-putty silicon paste (Cub Kit Profle, Vigodent) to serve as a standard guide for the tip of the spectrophotometer. A window was created on the buccal surface of the silicone guide toward the right maxillary canine, using a metal device approximately 6 mm in diameter (punch). A calibrated evaluator measured the color in all participants using a spectrophotometer (VITA Easyshade Advance, Vita Zahnfabrik) at the beginning of the first session and 30 days after the end of the bleaching treatment.
The objective color change was measured after the CIELab parameters of L* (luminosity), a* (green to red axis), and b* (blue to yellow axis) were obtained from the spectrophotometer. The difference between the baseline and 30 days after the end of the bleaching treatment was computed using the following CIELab formula [46]: ∆Eab = [(∆L*)2 + (∆a*)2 + (∆b*)2]1/2. In addition, the color change was also calculated based on the CIEDE 2000 formula [47]: ∆E00 = [(ΔL /kLSL)2 + (ΔC/ kCSC)2 + (ΔH/kHSH)2 + RT (ΔC × ΔH/SC × SH)]1/2 and whiteness index [48]: ΔWID = (0.511L*) − (2.3424a*) − (1.100b*).
Statistical analysis
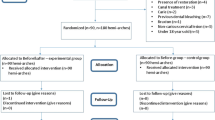
The statistician was blinded to the groups. We performed both the intention-to-treat analysis (as planned a priori) and the per-protocol analysis. All randomized participants were incorporated into the data set in the intention-to-treat analysis. In contrast, in the per-protocol analysis, we excluded patients who did not perform the two bleaching sessions (Fig. 1).
The absolute risks of the TS of both groups were compared using McNemar’s exact test (α = 0.05, test for proportion of dependent data ratio). Then, the odds risk and the 95% confidence interval (CI) were calculated.
The assumptions of the normal distribution (Kolmogorov–Smirnov test) and equal variance (Barlett’s test) of the continuous data sets were inspected. We used the Wilcoxon signed-rank test to compare the TS intensity in the NRS scale, and we used the paired t-test to compare data from the VAS scale. The subjective color assessment (ΔSGUs) and objective color assessment (ΔEab, ΔE00, and ΔWID) were compared with a paired t-test. The mean difference and 95% CI were also calculated as the effect measures for the continuous outcomes.
We calculated Spearman’s correlation between the two groups’ TS risk paired data, and we also calculated the Pearson’s correlation between the TS intensity data for both groups in the different dental arches. The statistical analysis was conducted in the software (SigmaPlot 14.0, Systat Software Inc. San Jose, CA, USA) with a significance level of 5%.
Results
Of the 50 participants, two did not return to recall evaluations after the first bleaching session, whereas five did not return after the second session. For the analysis of color change (intention-to-treat analysis), missing data from the color change were imputed using the last-outcome-carried-forward (LOCF) approach. These seven patients were excluded from the data set in the per-protocol analysis. Both analyses yielded similar conclusions (not shown data), and we presented data from the intention-to-treat analysis.
As for the risk and intensity of TS, a modified intention-to-treat analysis was performed, as we did not have any data from two participants, which prevented us from making any imputation. The exclusion of data was equal in the study, as it was a split-mouth study. Thus, it is unlikely that this procedure introduced biases to the study findings.
These seven participants returned to their home cities and reportedly lost interest in doing the bleaching protocol.
Demographic features of the participants
Fifty-nine participants were examined, and a total of 50 participants were included in the clinical study (Fig. 1). The baseline color of canines in the Vita Classical shade guide units was very similar for both groups (experimental gel: 9.7 ± 2.8; placebo: 9.8 ± 2.8). The participants were predominantly young adults with a mean age of 23.4 ± 7.5 years. Most participants were female (60.5%).
Risk of tooth sensitivity
The majority of the participants (96%) felt some discomfort during treatment. Forty-five participants reported pain on the experimental arch side, and all of them also reported pain on the placebo hemiarch side. Two participants did not report pain on either hemiarch side. In relative terms, the odds ratio for pain was 0.65 (0.1 to 4.1; p = 1.0; Table 1). The Spearman correlation coefficient for the pairs of binary data was strong and significant (r = 0.80; p-value < 0.001).
Intensity of tooth sensitivity (TS)
The statistical analysis did not show any significant difference in the intensity of TS between the groups for any of the pain scales (p = 0.77 for NRS, and p = 0.25 for VAS; Table 2). The mean difference of the pain intensity on the VAS scale was, on average, 0.35 units lower, which is unlikely to be clinically important. The pain was positively correlated in both groups (Table 2). The correlation was strong and significant for both pain scales. For the NRS, the Spearman correlation was 0.76 (p < 0.001), and for the VAS, the Pearson correlation was 0.77 (p < 0.001).
Color evaluation
A significant color change occurred in all groups after bleaching, which was approximately, and on average, five units on the Vita Classical scale, five units on the Vita Bleachedguide, 15 units on the ∆Eab, nine units on the ∆E00, and nine units on the ∆WID (Table 3). No significant difference in color change was observed between the groups (Table 3; p > 0.32).
Discussion
The conducting of this clinical trial was met with some challenges in the final phase of the data collection. The color change analysis performed 30 days after bleaching coincided with the emergent COVID-19 pandemic, which prevented us from evaluating the last color change in this specific time assessment period. Thus, the final color assessment had to be done 2 to 6 months after bleaching for some patients. Still, it is unlikely to have introduced bias because the comparison of the immediate results (approximately 30 days after bleaching) and those obtained 3 to 12 months after bleaching did not report any statistical and clinical differences between these assessment periods [49,50,51,52,53].
A total of seven patients decided to discontinue the bleaching protocol, and for two of them, no data were collected. Therefore, any imputation could be misleading. Because the missing data were balanced between groups (paired study design) and not at the randomization level, we excluded these two patients from the data analysis. These two exclusions explain why we performed a modified intention-to-treat analysis for the TS outcomes. In this modified approach, we used data only from the patients we could extract data from both bleaching sessions (n = 43) or at least the first bleaching session (n = 5).
Contrary to our expectations, the experimental desensitizing gel did not cause any reduction of the risk and intensity of TS. We believed that a synergic effect could be observed with the combination of the different agents used in experimental desensitizing gel. While there are no studies demonstrating efficacy of topical use of dexamethasone, previously studies have demonstrated a significant reduction in TS when the topical application of glutaraldehyde, potassium nitrate, and calcium agents were used [25, 28, 29, 34, 36]. Such agents are the most effective to date regarding the reduction of TS. Therefore, our hypothesis was that the association of these agents, with different mechanisms of action, could promote an increase in their effectiveness to reduction of TS.
However, it was not possible to predict how the dexamethasone used was able to penetrate through enamel and dentin and reach the pulp to produce the desired effect. Likewise, other agents, like calcium gluconate and potassium nitrate, could be able to deposit in the surface of enamel and dentin structure and prevent the penetration of hydrogen peroxide. This may have impaired the results in our study, and future in vitro studies need to be done to evaluate this hypothesis.
On the other hand, it is possible that the concentration of the agents used was not sufficient to produce the anti-inflammatory effects expected for dexamethasone, and the saturation and interaction with components on the enamel surface expect for the calcium-containing agents. Still, the use of nanotechnology for the formulation of the desensitizing agent could have promoted more satisfactory results regarding TS, as already demonstrated in previous studies [54, 55]. The use of nanotechnology provides advantages such as therapeutic efficacy, prolonged drug release, decreased toxicity, and longer action time [56] which could favoring drug penetration through the tooth structure. Therefore, the use of nanotechnology in the production of desensitizing and obliterating agents may promote more promising results. Future studies should be conducted to confirm this information.
Although the exact mechanism of bleaching-induced TS has not yet been explained, it is likely due to the damage that HP causes to living tissues from pulp tissue [9, 57]. In the case of injury, an acute inflammatory response begins to remove damaged tissue components to allow the body to begin the healing process. Due to increased blood flow, blood vessels dilate and eventually increase their permeability [8, 58], thus allowing fluid, proteins, and white blood cells to migrate from the circulation to the site of the tissue damage. A study found a higher density of macrophages and infiltrate inflammatory in the pulps that underwent in-office bleaching with 38% HP [59]. Macrophages are involved in the degradation of the extracellular matrix, the recruitment of leukocytes and pro-inflammatory cytokines, neovascularization, and fibroblast proliferation, among others [60, 61].
The edema within pulp tissue that occurs due to the release of inflammatory mediators and blood cells is different from what occurs in other connective tissues. Pulp tissue behaves differently because it is unique in that its soft tissues (pulp and pulp-dentin complex) are enclosed within mineralized hard tissues [62]. A rich neurovascular network that regulates various inflammatory mediators supplies the pulp tissue [63]. Thus, any minimal inflammatory signals and mediators may progress to pain.
It was already demonstrated that HP could reach the pulp tissue 15 min after being applied on the buccal enamel [55, 64]. This may occur because HP is a small molecule with a molecular mass of 35.01 g/mol−1. The molecular mass of calcium gluconate (430.37 g/mol−1), dexamethasone acetate (434.50 g/mol−1), potassium nitrate (101.10 g/mol−1), and glutaraldehyde (100.11 g/mol−1) are higher than that of HP. They, therefore, may take longer to reach the pulp.
However, earlier clinical trials showed the beneficial effects of the desensitizing agents included in the experimental gel when used alone [24, 25, 28, 29, 65]. Most of these RCTs used low sample sizes (low study power) and a parallel design that did not control for intra-individual variability. The high correlation of the risk and TS intensity values between the dental hemiarches suggests that the split-mouth design can reduce the sample size while keeping the study power high enough to detect clinically meaningful differences.
When a total of 16 studies evaluating potassium-nitrate desensitizers were collected in a systematic review of the literature [26], a significant and positive effect in favor of the potassium nitrate was observed. Still, this effect was subtle and not clinically significant. Similarly, a recent RCT that evaluated the impact of the topical application of a corticoid-containing product did not find any significant reduction in the risk and intensity of TS [14, 21]. Similarly, the use of glutaraldehyde has not shown positive results in reducing TS when used alone [65]. However, the use of an experimental gel containing potassium nitrate and glutaraldehyde was able to reduce the risk and intensity of TS after in-office dental bleaching [25]. Thus, we can believe that the association of agents with different mechanisms of action could promote an increase in their effectiveness.
Altogether, this means that it is unlikely that the topical application of desensitizers can minimize bleaching-induced TS. More recently, another RCT showed promising results by associating topical bioactive desensitizers with intraoral drug prescription (acetaminophen/codeine) [34], but further studies should confirm these findings.
Another aspect of this study that we should not rule out is that the combination of these agents may impair each other’s action via unknown mechanisms. However, the experimental gel was prepared and applied soon after preparation, thus reducing the likelihood of this hypothesis.
The color change was observed for both hemiarches irrespectively of the groups and color evaluation tools employed. In the present study, we measured color change using both subjective methods (color guide units) and objective methods (spectrophotometer). Shade guide units can provide a direct clinical indication of the degree of whitening [67], and therefore, they are widely employed in RCTs involving bleaching.
An objective evaluation is less clinically tangible but allows for the collection of more information. Using the same parameters of L*, a*, and b* parameters, we could calculate color change using the conventional CIELab 76 system (ΔEab), the CIEDE2000 system (ΔE00), and the whiteness index for dentistry (ΔWID) [46,47,48]. The CIEDE2000 system has been more recently employed, as it better estimates the visual perception of color [68]. The whiteness index provided more information on the direction of the bleaching effect [48] and has been added to recent RCTs about bleaching [21, 50, 53, 66].
To translate the ΔE values to the clinical scenario, clinicians should compare them with the 50:50 perceptibility (PT) and 50:50 acceptability (AT) thresholds [66]. The PT value is the minimal color difference that human eyes can distinguish. On the other hand, the AT value is more comprehensive, representing an existing difference acceptable for most people. The 50:50 PT and AT values for ΔEab were reported to be 1.2 and 2.7, respectively [66], whereas for ΔE00, the values were 0.8 and 1.8, respectively [66]. By looking at Table 3, one can see that the difference in the means between the study groups did not reach these thresholds, so they are clinically unimportant. On the other hand, these thresholds were exceeded in all of the time assessment periods, which is evidence of effective whitening.
Finally, in relation to the limiting factors of this study, we need to mention the fact that most of the participants were young adults, which may affect the generalization of the results for the general population. In addition, the subjectivity at the time of reporting pain may lead to changes in the observed results. Also, it is not known whether the concentration of the agents used was sufficient to produce the expected effect, which also leads to the need for further studies.
Conclusions
The application of an experimental desensitizer (calcium gluconate, dexamethasone acetate, potassium nitrate, and glutaraldehyde) before in-office bleaching did not reduce the risk and the intensity of tooth sensitivity and did not affect color change.
References
Bonafé E, Rezende M, Machado MM et al (2021) Personality traits, psychosocial effects and quality of life of patients submitted to dental bleaching. BMC Oral Health 21:7. https://doi.org/10.1186/s12903-020-01370-6
Goettems ML, Fernandez MDS, Donassollo TA et al (2021) Impact of tooth bleaching on oral health-related quality of life in adults: a triple-blind randomised clinical trial. J Dent 105:103564. https://doi.org/10.1016/j.jdent.2020.103564
Heymann HO (2005) Tooth whitening: facts and fallacies. Br Dent J 198:514. https://doi.org/10.1038/sj.bdj.4812298
Perdigão J (2010) Dental whitening–revisiting the myths. Northwest Dent 89(19–21):23–16
Maran BM, Matos TP, de Castro ADS et al (2020) In-office bleaching with low/medium vs. high concentrate hydrogen peroxide: a systematic review and meta-analysis. J Dent 103:103499. https://doi.org/10.1016/j.jdent.2020.103499
de Geus JL, Wambier LM, Kossatz S et al (2016) At-home vs in-office bleaching: a systematic review and meta-analysis. Oper Dent 41:341–356. https://doi.org/10.2341/15-287-lit
Kwon SR, Wertz PW (2015) Review of the mechanism of tooth whitening. J Esthet Restor Dent 27:240–257. https://doi.org/10.1111/jerd.12152
Roderjan DA, Stanislawczuk R, Hebling J et al (2015) Response of human pulps to different in-office bleaching techniques: preliminary findings. Braz Dent J 26:242–248. https://doi.org/10.1590/0103-6440201302282
Cintra LT, Benetti F, da Silva Facundo AC et al (2013) The number of bleaching sessions influences pulp tissue damage in rat teeth. J Endod 39:1576–1580. https://doi.org/10.1016/j.joen.2013.08.007
Caviedes-Bucheli J, Muñoz HR, Azuero-Holguín MM, Ulate E (2008) Neuropeptides in dental pulp: the silent protagonists. J Endod 34:773–788. https://doi.org/10.1016/j.joen.2008.03.010
Le Fur-Bonnabesse A, Bodéré C, Hélou C, Chevalier V, Goulet JP (2017) Dental pain induced by an ambient thermal differential: pathophysiological hypothesis. Pain 10:2845–2851. https://doi.org/10.2147/jpr.S142539
Piknjač A, Soldo M, Illeš D, KnezovićZlatarić D (2021) Patients’ assessments of tooth sensitivity increase one day following different whitening treatments. Acta Stomatol Croat 55:280–290. https://doi.org/10.15644/asc55/3/5
Coppla FM, Rezende M, de Paula E et al (2018) Combination of acetaminophen/codeine analgesics does not avoid bleaching-induced tooth sensitivity: a randomized, triple-blind two-center clinical trial. Oper Dent 43:E53-e63. https://doi.org/10.2341/17-092-c
Rezende M, Chemin K, Vaez SC et al (2018) Effect of topical application of dipyrone on dental sensitivity reduction after in-office dental bleaching: a randomized, triple-blind multicenter clinical trial. J Am Dent Assoc 149:363–371. https://doi.org/10.1016/j.adaj.2017.11.003
de Paula EA, Loguercio AD, Fernandes D et al (2013) Perioperative use of an anti-inflammatory drug on tooth sensitivity caused by in-office bleaching: a randomized, triple-blind clinical trial. Clin Oral Investig 17:2091–2097. https://doi.org/10.1007/s00784-013-0918-2
Fernandes MT, Vaez SC, Lima CM et al (2017) Preemptive use of naproxen on tooth sensitivity caused by in-office bleaching: a triple-blind, crossover, randomized clinical trial. Oper Dent 42:486–496. https://doi.org/10.2341/16-100-c
Paula E, Kossatz S, Fernandes D et al (2013) The effect of perioperative ibuprofen use on tooth sensitivity caused by in-office bleaching. Oper Dent 38:601–608. https://doi.org/10.2341/12-107-c
Silva KL, Sutil E, Hortkoff D et al (2021) Coadministration of ibuprofen/caffeine on bleaching-induced tooth sensitivity: a randomized clinical trial. Braz Dent J 32:105–115. https://doi.org/10.1590/0103-6440202104138
de Paula EA, Kossatz S, Fernandes D et al (2014) Administration of ascorbic acid to prevent bleaching-induced tooth sensitivity: a randomized triple-blind clinical trial. Oper Dent 39:128–135. https://doi.org/10.2341/12-483-c
da Costa Poubel LA, de Gouvea CVD, Calazans FS et al (2019) Pre-operative use of dexamethasone does not reduce incidence or intensity of bleaching-induced tooth sensitivity. A triple-blind, parallel-design, randomized clinical trial. Clin Oral Investig 23:435–444. https://doi.org/10.1007/s00784-018-2452-8
Favoreto MW, Vochikovski L, Terra RMO et al (2021) Topical application of Otosporin® before in-office bleaching: a split mouth, triple-blind, multicenter randomized clinical trial. Clin Oral Investig. https://doi.org/10.1007/s00784-021-04224-9
Rezende M, Bonafé E, Vochikovski L et al (2016) Pre- and postoperative dexamethasone does not reduce bleaching-induced tooth sensitivity: a randomized, triple-masked clinical trial. J Am Dent Assoc 147:41–49. https://doi.org/10.1016/j.adaj.2015.07.003
Carregosa Santana ML, Leal PC, Reis A, Faria e Silva AL (2019) Effect of anti-inflammatory and analgesic drugs for the prevention of bleaching-induced tooth sensitivity: a systematic review and meta-analysis. J Am Dent Assoc 150:818 829-e814. https://doi.org/10.1016/j.adaj.2019.05.004
Mehta D, Venkata S, Naganath M et al (2013) Clinical trial of tooth desensitization prior to in-office bleaching. Eur J Oral Sci 121:477–481. https://doi.org/10.1111/eos.12067
Parreiras SO, Szesz AL, Coppla FM et al (2018) Effect of an experimental desensitizing agent on reduction of bleaching-induced tooth sensitivity: a triple-blind randomized clinical trial. J Am Dent Assoc 149:281–290. https://doi.org/10.1016/j.adaj.2017.10.025
Martini EC, Favoreto MW, Rezende M et al (2021) Topical application of a desensitizing agent containing potassium nitrate before dental bleaching: a systematic review and meta-analysis. Clin Oral Investig 25:4311–4327. https://doi.org/10.1007/s00784-021-03994-6
Martini EC, Parreiras SO, Szesz AL et al (2020) Bleaching-induced tooth sensitivity with application of a desensitizing gel before and after in-office bleaching: a triple-blind randomized clinical trial. Clin Oral Investig 24:385–394. https://doi.org/10.1007/s00784-019-02942-9
Mehta D, Jyothi S, Moogi P et al (2018) Novel treatment of in-office tooth bleaching sensitivity: a randomized, placebo-controlled clinical study. J Esthet Restor Dent 30:254–258. https://doi.org/10.1111/jerd.12374
Oldoini G, Bruno A, Genovesi AM, Parisi L (2018) Effects of amorphous calcium phosphate administration on dental sensitivity during in-office and at-home interventions. Dent J (Basel). https://doi.org/10.3390/dj6040052
Rezende M, da Silva KL, Miguel TC et al (2020) Prior application of 10% potassium nitrate to reduce postbleaching sensitivity: a randomized triple-blind clinical trial. J Evid Based Dent Pract 20:101406. https://doi.org/10.1016/j.jebdp.2020.101406
Ibrahim I MA, El Banna MJLSJ (2011) Evaluation of the sustainability of different desensitizing agents after in-office bleaching. Life Sci J 8:121–134
Arrais CA, Chan DC, Giannini M (2004) Effects of desensitizing agents on dentinal tubule occlusion. J Appl Oral Sci 12:144–148. https://doi.org/10.1590/s1678-77572004000200012
Parreiras SO, Favoreto MW, Lenz RE et al (2020) Effect of prior application of desensitizing agent on the teeth submitted to in-office bleaching. Braz Dent J 31:236–243. https://doi.org/10.1590/0103-6440202003365
de Araújo IDT, de Sousa S K, das Neves Peixoto TVO et al (2021) The combined use of systemic analgesic/anti-inflammatory drugs and a bioactive topical desensitizer for reduced in-office bleaching sensitivity without jeopardizing the hydrogen peroxide efficacy: a randomized, triple blinded, split-mouth clinical trial. Clin Oral Investig 25:6623–6632. https://doi.org/10.1007/s00784-021-03948-y
Kossatz S, Martins G, Loguercio AD, Reis A (2012) Tooth sensitivity and bleaching effectiveness associated with use of a calcium-containing in-office bleaching gel. J Am Dent Assoc 143:e81-87. https://doi.org/10.14219/jada.archive.2012.0075
Maghaireh GA, Alzraikat H, Guidoum A (2014) Assessment of the effect of casein phosphopeptide-amorphous calcium phosphate on postoperative sensitivity associated with in-office vital tooth whitening. Oper Dent 39:239–247. https://doi.org/10.2341/12-527-c
Oksa M, Haapanen A, Furuholm J et al (2021) Effect of perioperative systemic dexamethasone on pain, edema, and trismus in mandibular fracture surgery: a randomized trial. J Craniofac Surg 32:2611–2614. https://doi.org/10.1097/scs.0000000000007775
Momesso GAC, Grossi-Oliveira GA, Silva WPP et al (2021) A triple-blind randomized clinical trial of different associations between dexamethasone and non-steroids anti-inflammatories for preemptive action in third molar extractions. Sci Rep 11:24445. https://doi.org/10.1038/s41598-021-04068-z
Oliveira EM, Oliveira VB, Araújo LK et al (2021) Anti-inflammatory effectiveness of oral dexamethasone 4 mg on mandibular third molar surgeries: a split-mouth randomized clinical trial. J Oral Maxillofac Surg 79:981–988. https://doi.org/10.1016/j.joms.2021.01.003
Aksoy F, Ege B, Tosun S (2021) The effect of pre-operative submucosal administration of dexamethasone, tramadol, articaine on the success rate of inferior alveolar nerve block on mandibular molars with symptomatic irreversible pulpitis: a randomized, double-blind placebo-controlled clinical trial. Int Endod J 54:1982–1992. https://doi.org/10.1111/iej.13604
Kumar M, Singla R, Gill GS, Kalra T, Jain N (2021) Evaluating combined effect of oral premedication with ibuprofen and dexamethasone on success of inferior alveolar nerve block in mandibular molars with symptomatic irreversible pulpitis: a prospective, double-blind, randomized clinical trial. J Endod 47:705–710. https://doi.org/10.1016/j.joen.2021.01.005
Patil RH, Naveen Kumar M, Kiran Kumar KM et al (2018) Dexamethasone inhibits inflammatory response via down regulation of AP-1 transcription factor in human lung epithelial cells. Gene 645:85–94. https://doi.org/10.1016/j.gene.2017.12.024
Pandis N, Chung B, Scherer RW et al (2019) CONSORT 2010 statement: extension checklist for reporting within person randomised trials. Br J Dermatol 180:534–552. https://doi.org/10.1111/bjd.17239
Maran BM, Vochikovski L, Hortkoff DRA et al (2020) Bleaching sensitivity with a desensitizing in-office bleaching gel: a randomized double-blind clinical trial. Quintessence Int 51:788–797. https://doi.org/10.3290/j.qi.a45173
Sutil E, da Silva KL, Terra RMO et al (2020) Effectiveness and adverse effects of at-home dental bleaching with 37% versus 10% carbamide peroxide: a randomized, blind clinical trial. J Esthet Restor Dent. https://doi.org/10.1111/jerd.12677
de l’Eclairage CIJPC (1978) Recommendations on uniform color spaces, color-difference equations, psychometric color terms.
Luo MR, Cui G, Rigg BJCR (2001) The development of the CIE 2000 colour-difference formula: CIEDE2000. Color Res Appl 26:340–350. https://doi.org/10.1002/col.1049
Pérez Mdel M, Ghinea R, Rivas MJ et al (2016) Development of a customized whiteness index for dentistry based on CIELAB color space. Dent Mater 32:461–467. https://doi.org/10.1016/j.dental.2015.12.008
Abrantes PS, Xavier CM, Melo A et al (2021) Efficacy, longevity, and bleaching sensitivity of carbamide and hydrogen peroxides for in-office bleaching: a 6-month randomized, double blind, split-mouth clinical trial. Am J Dent 34:17–22
Bersezio C, Martín J, Prieto MV et al (2019) One-year bleaching efficacy using two HP products with different pH: a double-blind randomized clinical trial. J Esthet Restor Dent 31:493–499. https://doi.org/10.1111/jerd.12505
Kim HJ, Jang JH, Choi D et al (2020) Bleaching toothpaste with two different concentrations of hydrogen peroxide: a randomized double-blinded clinical trial. J Dent 103:103508. https://doi.org/10.1016/j.jdent.2020.103508
Kury M, Wada EE, da Silva PS et al (2022) Colorimetric evaluation after in-office tooth bleaching with violet LED: 6- and 12-month follow-ups of a randomized clinical trial. Clin Oral Investig 26:837–847. https://doi.org/10.1007/s00784-021-04062-9
Martini EC, Favoreto MW, de Andrade HF et al (2021) One-year follow-up evaluation of reservoirs in bleaching trays for at-home bleaching. J Esthet Restor Dent 33:992–998. https://doi.org/10.1111/jerd.12797
Browning WD, Cho SD, Deschepper EJ (2012) Effect of a nano-hydroxyapatite paste on bleaching-related tooth sensitivity. J Esthet Restor Dent 24:268–276. https://doi.org/10.1111/j.1708-8240.2011.00437.x
Favoreto MW, Madureira MP, Hass V et al (2021) A novel carbamide peroxide polymeric nanoparticle bleaching gel: color change and hydrogen peroxide penetration inside the pulp cavity. J Esthet Restor Dent 33:277–283. https://doi.org/10.1111/jerd.12652
Uchegbu IF (2006) Pharmaceutical nanotechnology: polymeric vesicles for drug and gene delivery. Expert Opin Drug Deliv 3:629–640. https://doi.org/10.1517/17425247.3.5.629
Kawamoto K, Tsujimoto Y (2004) Effects of the hydroxyl radical and hydrogen peroxide on tooth bleaching. J Endod 30:45–50. https://doi.org/10.1097/00004770-200401000-00010
Ferreira VG, Nabeshima CK, Marques MM et al (2013) Tooth bleaching induces changes in the vascular permeability of rat incisor pulps. Am J Dent 26:298–300
Vaz MM, Lopes LG, Cardoso PC et al (2016) Inflammatory response of human dental pulp to at-home and in-office tooth bleaching. J Appl Oral Sci 24:509–517. https://doi.org/10.1590/1678-775720160137
Koh TJ, DiPietro LA (2011) Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med 13:e23. https://doi.org/10.1017/s1462399411001943
Wisithphrom K, Murray PE, Windsor LJ (2006) Interleukin-1 alpha alters the expression of matrix metalloproteinases and collagen degradation by pulp fibroblasts. J Endod 32:186–192. https://doi.org/10.1016/j.joen.2005.10.055
Van Hassel HJ (2021) Reprint of: Physiology of the human dental pulp. J Endod 47:690–695. https://doi.org/10.1016/j.joen.2021.03.001
Cooper JS, Bokmeyer TJ, Bowles WH (1992) Penetration of the pulp chamber by carbamide peroxide bleaching agents. J Endod 18:315–317. https://doi.org/10.1016/s0099-2399(06)80479-6
Tay LY, Kose C, Loguercio AD, Reis A (2009) Assessing the effect of a desensitizing agent used before in-office tooth bleaching. J Am Dent Assoc 140:1245–1251. https://doi.org/10.14219/jada.archive.2009.0047
Diniz A, Lima S, Tavarez R, Borges AH et al (2018) Preventive use of a resin-based desensitizer containing glutaraldehyde on tooth sensitivity caused by in-office bleaching: a randomized, single-blind clinical trial. Oper Dent 43:472–481. https://doi.org/10.2341/17-020-c]
Paravina RD, Ghinea R, Herrera LJ et al (2015) Color difference thresholds in dentistry. J Esthet Restor Dent 27:S1-9. https://doi.org/10.1111/jerd.12149]
Pecho OE, Ghinea R, Alessandretti R et al (2016) Visual and instrumental shade matching using CIELAB and CIEDE2000 color difference formulas. Dent Mater 32:82–92. https://doi.org/10.1016/j.dental.2015.10.015
Meireles SS, de Oliveira RDB, Barbosa MTG, da Silva KL, Loguercio AD (2022) Efficacy and tooth sensitivity of at-home bleaching in patients with esthetic restorations: a randomized clinical trial. Clin Oral Invest 26:565–573. https://doi.org/10.1007/s00784-021-04035-y
Acknowledgements
This study was performed by Laína Vochikovski as partial fulfillment of her PhD degree at the State University of Ponta Grossa (UEPG), Ponta Grossa, PR, Brazil. The authors would like to thank FGM Dental Products for the donation of the bleaching gel used in this investigation.
Funding
This study was partially supported by the National Council for Scientific and Technological Development (CNPq) under grants 304817/2021–0 and 308286/2019–7 and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Contributions
Conceptualization [Laína Vochikovski, Marcia Rezende, Paulo Vitor Farago, Alessandro Dourado Loguercio, Alessandra Reis]; Methodology [Laína Vochikovski, Michael Willian Favoreto, Marcia Rezende, Renata Maria Olenki Terra, Karine Letícia da Silva, Alessandra Reis]; Investigation [Laína Vochikovski, Michael Willian Favoreto, Marcia Rezende, Renata Maria Olenki Terra, Karine Letícia da Silva, Alessandra Reis]; Resources [Alessandro Dourado Loguercio, Alessandra Reis]; Data Curation [Laína Vochikovski, Michael Willian Favoreto, Marcia Rezende, Renata Maria Olenki Terra, Karine Letícia da Silva, Alessandra Reis]; Formal analysis [Michael Willian Favoreto, Paulo Vitor Farago, Alessandro Dourado Loguercio, Alessandra Reis]; Writing –Original Draft [Laína Vochikovski, Michael Willian Favoreto, Marcia Rezende, Renata Maria Olenki Terra, Karine Letícia da Silva]; Writing –Review & Editing [Paulo Vitor Farago, Alessandro Dourado Loguercio, Alessandra Reis]; Supervision [Alessandro Dourado Loguercio, Alessandra Reis]; Project administration [Alessandra Reis].
Corresponding author
Ethics declarations
Ethical approval
The clinical investigation was approved (protocol 3.893.891) by the scientific review committee and by the committee for the protection of human participants of the State University of Ponta Grossa/PR/Brasil.
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no competing interests.
Informed consent
All participants gave their informed consent prior to their inclusion in the study. Details that might disclose the identity of the subjects under study were omitted. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vochikovski, L., Favoreto, M.W., Rezende, M. et al. Effect of an experimental desensitizing gel on bleaching-induced tooth sensitivity after in-office bleaching—a double-blind, randomized controlled trial. Clin Oral Invest 27, 1567–1576 (2023). https://doi.org/10.1007/s00784-022-04778-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-022-04778-2