Abstract
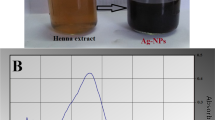
Investigating the application of innovative antimicrobial surface coatings on medical devices is an important field of research. Many of these coatings have significant drawbacks, including biocompatibility, coating stability and the inability to effectively combat multiple drug-resistant bacteria. In this research, we developed an antibiofilm surface coating for medical catheters using biosynthesized silver nanoparticles (b-Cs-AgNPs) developed using leaves extract of Calliandra surinamensis. Various characterization techniques were employed to thoroughly characterize the synthesized b-Cs-AgNPs and c-AgNPs. b-Cs-AgNPs were compatible with human normal kidney cells and chicken embryos. It did not trigger any skin inflammatory response in in vivo rat model. b-Cs-AgNPs demonstrated potent zone of inhibition of 19.09 mm when subjected to the disc diffusion method in E. coli confirming strong antibacterial property. Different anti-bacterial assays including liquid growth curve, colony counting assay, biofilm formation assay supported the potent antimicrobial efficacy of b-Cs-AgNPs alone and when coated to medical grade catheters. Mechanistic studies reveal the presence of ferulic acid, that was important for the synthesis of b-AgNPs along with enhanced antibacterial effects of b-Cs-AgNPs compared to c-AgNPs, supported by molecular docking analysis. These results together demonstrated the effective role b-Cs-AgNPs in combating infections and mitigating biofilm formations, highlighting their need for further study in the field of biomedical applications.
Graphical abstract
Schematic Illustration of Eco-Friendly Synthesis for Biofilm Prevention on Medical Catheters and Bacterial Infection Mitigation. Created with BioRender.com.









Similar content being viewed by others
Data availability
All data related to the manuscript will be readily available upon request.
References
Bhardwaj T, Ramana LN, Sharma TK (2022) Current advancements and future road map to develop ASSURED microfluidic biosensors for infectious and non-infectious diseases. Biosensors 12(5):357
Arney D, Venkatasubramanian KK, Sokolsky O, Lee I (2011) Biomedical devices and systems security. In: 2011 Annual international conference of the IEEE engineering in medicine and biology society, 2011, pp. 2376–2379
Magill SS et al (2014) Multistate point-prevalence survey of health care-associated infections (in eng). N Engl J Med 370(13):1198–1208
Noimark S, Dunnill CW, Wilson M, Parkin IP (2009) The role of surfaces in catheter-associated infections (in eng). Chem Soc Rev 38(12):3435–3448
Burroughs L, Ashraf W, Singh S, Martinez-Pomares L, Bayston R, Hook AL (2020) Development of dual anti-biofilm and anti-bacterial medical devices (in eng). Biomater Sci 8(14):3926–3934
Keum H et al (2017) Prevention of bacterial colonization on catheters by a one-step coating process involving an antibiofouling polymer in water. ACS Appl Mater Interfaces 9(23):19736–19745
Francolini I, Donelli G, Stoodley P (2003) Polymer designs to control biofilm growth on medical devices. Rev Environ Sci Biotechnol 2(2):307–319
Gwisai T et al (2017) Repurposing niclosamide as a versatile antimicrobial surface coating against device-associated, hospital-acquired bacterial infections (in eng). Biomed Mater 12(4):045010
Cloutier M, Mantovani D, Rosei F (2015) Antibacterial coatings: challenges, perspectives, and opportunities. Trends Biotechnol 33(11):637–652
Ding X et al (2012) Antibacterial and antifouling catheter coatings using surface grafted PEG-b-cationic polycarbonate diblock copolymers. Biomaterials 33(28):6593–6603
Woo J et al (2020) Facile synthesis and coating of aqueous antifouling polymers for inhibiting pathogenic bacterial adhesion on medical devices. Prog Org Coat 147:105772
Sohns JM, Bavendiek U, Ross TL, Bengel FM (2017) Targeting cardiovascular implant infection: multimodality and molecular imaging (in eng). Circ Cardiovasc Imaging 10(12)
Devine R, Singha P, Handa H (2019) Versatile biomimetic medical device surface: hydrophobin coated, nitric oxide-releasing polymer for antimicrobial and hemocompatible applications. Biomater Sci 7(8):3438–3449. https://doi.org/10.1039/C9BM00469F
Damodaran VB, Murthy NS (2016) Bio-inspired strategies for designing antifouling biomaterials (in eng). Biomater Res 20:18
Zheng W, Jia Y, Chen W, Wang G, Guo X, Jiang X (2017) Universal coating from electrostatic self-assembly to prevent multidrug-resistant bacterial colonization on medical devices and solid surfaces (in eng). ACS Appl Mater Interfaces 9(25):21181–21189
Martinez-Gutierrez F et al (2013) Anti-biofilm activity of silver nanoparticles against different microorganisms. Biofouling 29(6):651–660
Loo C-Y, Young PM, Lee W-H, Cavaliere R, Whitchurch CB, Rohanizadeh R (2014) Non-cytotoxic silver nanoparticle-polyvinyl alcohol hydrogels with anti-biofilm activity: designed as coatings for endotracheal tube materials. Biofouling 30(7):773–788
Ashmore DA et al (2018) Evaluation of E. coli inhibition by plain and polymer-coated silver nanoparticles. Revista do Instituto de Medicina Tropical de São Paulo 60:e18.
Sahoo B et al (2023) Photocatalytic activity of biosynthesized silver nanoparticle fosters oxidative stress at nanoparticle interface resulting in antimicrobial and cytotoxic activities. Environ Toxicol
Campo-Beleño C et al (2022) Biologically synthesized silver nanoparticles as potent antibacterial effective against multidrug-resistant Pseudomonas aeruginosa. Lett Appl Microbiol 75(3):680–688
Mukherjee S et al (2014) Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 4(3):316
Rajan R, Chandran K, Harper SL, Yun S-I, Kalaichelvan PT (2015) Plant extract synthesized silver nanoparticles: an ongoing source of novel biocompatible materials. Indust Crops Prod 70:356–373
Omar SM, Ahmat N, Nik Azmin NF, Sabandar CW, Muqarrabun A, Ramadhan LM (2013) Isolation and characterization of chemical constituents from the flower of Calliandra surinamensis benth. Open Conf Proc J 4(1)
Falodun A, Irabor EE (2008) Phytochemical, proximate, antioxidant and free radical scavenging evaluations of Calliandria surinamensis (in eng). Acta Pol Pharm 65(5):571–575
Procópio TF et al (2017) CasuL: a new lectin isolated from Calliandra surinamensis leaf pinnulae with cytotoxicity to cancer cells, antimicrobial activity and antibiofilm effect. Int J Biol Macromol 98:419–429
Alzahrani A, Abbott G, Young L, Igoli J, Gray A, Ferro V (2016) Phytochemical and biological investigation of Calliandra surinamensis as a potential treatment for diabetes. Planta Med 82(Suppl. 01):P385
Somba GC, Edi HJ, Siampa JP (2019) Formulasi sediaan krim ekstrak etanol daun kaliandra (Calliandra surinamensis) dan uji aktivitas antibakterinya terhadap bakteri Staphylococcus aureus. Pharmacon 8(4):809–814
Domitrović R, Rashed K, Cvijanović O, Vladimir-Knežević S, Škoda M, Višnić A (2015) Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice (in eng). Chem Biol Interact 230:21–29
Saleem M (2009) Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett 285(2):109–115
Pirtarighat S, Ghannadnia M, Baghshahi S (2019) Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J Nanostruct Chem 9(1):1–9
Patra S, Mukherjee S, Barui AK, Ganguly A, Sreedhar B, Patra CR (2015) Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater Sci Eng C 53:298–309
Xiao F, Xu T, Lu B, Liu R (2020) Guidelines for antioxidant assays for food components. Food Front 1(1):60–69
Chowdhury SR, Mukherjee S, Das S, Patra CR, Iyer PK (2017) Multifunctional (3-in-1) cancer theranostics applications of hydroxyquinoline-appended polyfluorene nanoparticles. Chem Sci 8(11):7566–7575. https://doi.org/10.1039/C7SC03321D
Keshari AK, Srivastava R, Singh P, Yadav VB, Nath G (2020) Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J Ayurveda Integr Med 11(1):37–44
García-Gareta E, Binkowska J, Kohli N, Sharma V (2020) Towards the development of a novel ex ovo model of infection to pre-screen biomaterials intended for treating chronic wounds. J Funct Biomater 11(2):37
Mukherjee S et al (2023) Screening hydrogels for antifibrotic properties by implanting cellularly barcoded alginates in mice and a non-human primate. Nat Biomed Eng 7(7):867–886
Kragh KN, Alhede M, Kvich L, Bjarnsholt T (2019) Into the well—a close look at the complex structures of a microtiter biofilm and the crystal violet assay. Biofilm 1:100006
Siddique MH et al. (2020) Effect of silver nanoparticles on biofilm formation and EPS production of multidrug-resistant Klebsiella pneumoniae. BioMed Res Int 6398165
Laskowski RA, Swindells MB (2011) LigPlot+: multiple ligand–protein interaction diagrams for drug discover. ACS Publications
Kerker M (1985) The optics of colloidal silver: something old and something new. J Colloid Interface Sci 105(2):297–314
Magdassi S, Bassa A, Vinetsky Y, Kamyshny A (2003) Silver nanoparticles as pigments for water-based ink-jet inks. Chem Mater 15(11):2208–2217
Kotcherlakota R et al (2019) Biosynthesized gold nanoparticles: in vivo study of near-infrared fluorescence (NIR)-based bio-imaging and cell labeling applications. ACS Biomater Sci Eng 5(10):5439–5452
Allaka G, King MFL, Yepuri V, Narayana RL (2023) Synthesis of silver oxide nanoparticles using gomutra mediation and their investigations on anti-oxidant property. Mater Today: Proc
Korkmaz N, Karadağ A (2021) Microwave assisted green synthesis of Ag, Ag2O, and Ag2O3 nanoparticles. J Turkish Chem Soc Sect A: Chem 8(2):585–592
He Y et al (2017) Green synthesis of silver nanoparticles using seed extract of Alpinia katsumadai, and their antioxidant, cytotoxicity, and antibacterial activities. RSC Adv 7(63):39842–39851. https://doi.org/10.1039/C7RA05286C
Mochalin V, Osswald S, Gogotsi Y (2009) Contribution of functional groups to the Raman spectrum of nanodiamond powders. Chem Mater 21(2):273–279
Allafchian AR, Mirahmadi-Zare SZ, Jalali SAH, Hashemi SS, Vahabi MR (2016) Green synthesis of silver nanoparticles using phlomis leaf extract and investigation of their antibacterial activity. J Nanostruct Chem 6(2):129–135
Konappa N et al (2021) Ameliorated antibacterial and antioxidant properties by Trichoderma harzianum mediated green synthesis of silver nanoparticles. Biomolecules 11(4):535
Bassetti S, Tschudin-Sutter S, Egli A, Osthoff M (2022) Optimizing antibiotic therapies to reduce the risk of bacterial resistance. Eur J Intern Med 99:7–12
Ahmad SA et al (2020) Bactericidal activity of silver nanoparticles: a mechanistic review. Mater Sci Energy Technol 3:756–769
Singh S, Singh SK, Chowdhury I, Singh R (2017) Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents (in eng). Open Microbiol J 11:53–62
Bala Subramaniyan S, Senthilnathan R, Arunachalam J, Anbazhagan V (2020) Revealing the significance of the glycan binding property of Butea monosperma seed lectin for enhancing the antibiofilm activity of silver nanoparticles against uropathogenic Escherichia coli. Bioconjugate Chem 31(1):139–148
Mikhlif H (2019) Ecofriendly route for waste upcycling and silver nanoparticles synthesis from citrus reticulata Peel. Int J Nanoelectron Mater 12:349–356
Procópio TF et al (2018) Calliandra surinamensis lectin (CasuL) does not impair the functionality of mice splenocytes, promoting cell signaling and cytokine production. Biomed Pharmacother 107:650–655
McConkey BJ, Sobolev V, Edelman M (2022) The performance of current methods in ligand–protein docking. Curr Sci 845–856
Borges A, Ferreira C, Saavedra MJ, Simões M (2013) Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb Drug Resist 19(4):256–265
Gross CH et al (2003) Active-site residues of Escherichia coli DNA gyrase required in coupling ATP hydrolysis to DNA supercoiling and amino acid substitutions leading to novobiocin resistance (in eng). Antimicrob Agents Chemother 47(3):1037–1046
Whaley SG, Rock CO (2020) Alternate fatty acid synthesis initiation in Escherichia coli. FASEB J 34(S1):1–1
Scotti R, Casciaro B, Stringaro A, Maggi F, Colone M, Gabbianelli R (2024) Fighting microbial infections from Escherichia coli O157: H7: the combined use of three essential oils of the cymbopogon genus and a derivative of Esculentin-1a peptide. Antibiotics 13(1):86
Couturier M, Bahassi EM, Van Melderen L (1998) Bacterial death by DNA gyrase poisoning. Trends Microbiol 6(7):269–275
Lu X, Tang J, Zhang Z, Ding K (2015) Bacterial β-ketoacyl-acyl carrier protein synthase III (FabH) as a target for novel antibacterial agents design. Curr Med Chem 22(5):651–667
Wang X, Preston JF III, Romeo T (2004) The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J Bacteriol 186(9):2724–2734
Dhahi RM, Mikhlif HM (2019) Ecofriendly route for waste upcycling and silver nanoparticles synthesis from citrus reticulata Peel. Int J Nanoelectron Mater 12(3)
Meruvu H, Vangalapati M, Chippada SC, Bammidi SR (2011) Synthesis and characterization of zinc oxide nanoparticles and its antimicrobial activity against Bacillus subtilis and Escherichia coli. J Rasayan Chem 4(1):217–222
Shivashankarappa A, Sanjay KR (2020) Escherichia coli-based synthesis of cadmium sulfide nanoparticles, characterization, antimicrobial and cytotoxicity studies. Braz J Microbiol 51:939–948
Rautela A, Rani J (2019) Green synthesis of silver nanoparticles from Tectona grandis seeds extract: characterization and mechanism of antimicrobial action on different microorganisms. J Anal Sci Technol 10(1):1–10
Ahmad A et al (2017) The effects of bacteria-nanoparticles interface on the antibacterial activity of green synthesized silver nanoparticles. Microb Pathog 102:133–142
Gross CH et al (2003) Active-site residues of Escherichia coli DNA gyrase required in coupling ATP hydrolysis to DNA supercoiling and amino acid substitutions leading to novobiocin resistance. Antimicrobial Agents Chemother 47(3):1037–1046
Acknowledgements
LP and AU thank UGC, New Delhi for their junior research fellowship. Dr Sudip Mukherjee acknowledges director (Prof. Pramod Kumar Jain), Indian Institute of Technology (BHU) for providing research infrastructure and other facilities. SM further acknowledged research fund support from IIT (BHU) [OH-35-Other Capital/Miscellaneous Grant] and the Royal Society of Chemistry, UK (No. R22-2922732087) for providing funding support for this work. Authors acknowledges Vaishali Yadav and Dr. Bama Charan Mondal, Department of Zoology, Banaras Hindu University, Varanasi, UP, India for helping in the confocal microscopic analysis and generously providing GFP-E. coli, respectively.
Funding
IIT (BHU) [OH-35-Other Capital/Miscellaneous Grant] and the Royal Society of Chemistry, UK (No. R22-2922732087).
Author information
Authors and Affiliations
Contributions
LP and SM has conceptualized the idea of this manuscript. LP, PS, MN, GS, AI, PP have performed the experiments, developed the methodology, and validated the results. SM and LP has written the original draft of the manuscript and revised. SM has acquired the funding and supervised the overall work as the corresponding author.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Dermal skin irritation study was performed after IAEC approval (IIT(BHU)/IAEC/2023/068; Approval Date: February 9, 2023).
Declaration of generative AI in scientific writing
No AI support was taken during the preparation of the manuscript at any stage.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pradhan, L., Sah, P., Nayak, M. et al. Biosynthesized silver nanoparticles prevent bacterial infection in chicken egg model and mitigate biofilm formation on medical catheters. J Biol Inorg Chem 29, 353–373 (2024). https://doi.org/10.1007/s00775-024-02050-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-024-02050-4