Abstract
In recent years, the green synthesis of silver nanoparticles using various plant extracts has attracted great attention. This is because, these methods are simple, inexpensive and, eco-friendly. In this study, it was observed that silver ions were reduced by phlomis leaf extract after 5 min, leading to the formation of crystalline silver nanoparticles. Phlomis species is known as a rich source of flavonoids, phenylpropanoids and other phenolic compounds. The silver nanoparticles produced by the phlomis extract were characterized by different techniques including UV–vis spectrophotometry, X-ray diffraction, scanning electron microscopy (SEM), transmission electron microscopy (TEM), and FT-IR. The SEM and TEM results indicated that AgNPs were predominantly spherical in shape with an average particle size of 25 nm. In addition, the antibacterial activity of biologically synthesized nanopartilcles against Gram-positive (Staphyloccocus aureus and Bacillus cereus) and Gram-negative (Salmonella typhimurium and Escherichia coli) bacteria was proved. This study, therefore, showed that the phlomis leaf extract could be used for the green synthesis of silver nanoparticles with the appropriate antibacterial activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most important fields of research in nanotechnology is the synthesis of different nanoparticles such as silver, gold, iron, etc. [1–4]. There are various chemical and physical methods for the synthesis of metallic nanoparticles for example, one can mention reduction of solutions, photochemical reactions in reverse micelles, electrochemical reduction, heat evaporation and radiation assisted methods, among others. Physical and chemical methods have usually been successful in the synthesis of nanomaterials in large quantities in short periods of time, as well for specific size and shape. However, most of these methods are extremely expensive and they also involve the use of toxic, hazardous chemicals as the stabilizers which may pose potential environmental and biological risks [5–8].
In recent years, the use of biological methods for the synthesis of metallic nanoparticles has received considerable attention, because these are inexpensive and eco–friendly; also, they can be carried out in one step [9]. Green synthesis methods utilize miscellaneous biological natural substances such as microorganisms, whole plants, plant tissues and fruits, plant extracts, marine algae and micro–fluids for the reduction and stabilization of nanoparticles. Synthesis of nanoparticles using plant extracts has several advantages over other environmentally green synthesis methods, because plants are broadly distributed, readily scalable, easily available, safe to handle and less expensive [2].
Among diverse nanaoparticles, silver nanoparticles due to various properties such as catalysis, electrochemical conductivity and antimicrobial activity, can be used in different applications like biomedicine, agriculture, photo chemicals and food chemistry [10, 11]. Since ancient times, the silver has been known to be efficient against a wide range of microorganisms [12]. Nowadays, the most important applications of silver nanoparticles in biotechnology science correspond to their antibacterial and antifungal activities [13].
Phlomis is a perennial native herb plant belonging to Lamiaceae family with more than 100 species in the world. This genus plant is mostly grown in Turkey, Iran, Asia, North Africa and Europe. About 17 species of this plant are in Iran, of which 10 are endemic. A review of several studies shows that phlomis species have unique aromatic compounds, medicinal properties and antimicrobial activity [14, 15].
A large number of plant leaf extracts like Cinnamomum camphora [16], Chenopodium album [17], Stevia rebaudiana [18], Murraya koenigii [19], Annona squamosal [20], Desmodium gangeticum [21], Pulicaria glutinosa [22], Brucea javanica [23], Artocarpus heterophyllus [24], Caesalpinia coriaria [25], Abutilon indicum [26], Rosa indica [27], Tephrosia tinctoria [28] have been employed by several researchers for the synthesis of silver nanoparticles.
In this research, the leaf extract of phlomis was used for rapid, simple and biosynthetic synthesis of silver nanoparticles. Furthermore, green synthesis Ag nanoparticles were characterized by FT-IR, XRD, TEM, and SEM techniques. Finally, its antibacterial activity was investigated by the disk diffusion method.
Materials and methods
Materials
Silver nitrate was obtained from Merck Company for this study. All glassware were washed in dilute HNO3 acid and rinsed with distilled water and dried in a hot air oven before use. During the period of flowering and the vegetative phase, samples of phlomis Arial parts consisting of leaves, stems and flower buds were collected. By comparing the collected voucher specimen with that of a known identity available in the herbarium of the Department of Natural Resources, Isfahan University of Technology, Iran, the taxonomic identity of the plant was confirmed.
Microorganisms and growth conditions
In this study, Gram-negative bacteria like Escherichia coli (ATCC 35218) and Salmonella typhimurium (ATCC 14028) and Gram-positive bacteria like Staphylococcus aureus (ATCC 29213) and Bacillus cereus (ATCC 14579) were employed as bacteria strains for the antimicrobial experiments. These bacteria strains were cultured at 37 °C on Luria-Bertani (LB) agar.
Preparation of the plant extract
The phlomis leaf extract was employed to prepare silver nanoparticles. The aerial parts of the fresh collected plant were washed several times with distilled water, to remove dust particles and any dirt. Leaves of the plants were dried in the shade and at room temperature. About 40 g of its leaves was subsequently ground into fine powder and boiled for 2 h in 250 ml of distilled water. The extracts were cooled to room temperature and filtered through whatman filter paper No. 1. The filtered extract was stored in refrigerator at 4 °C until it was used as the reducing and stabilizing agent.
Synthesis of silver nanoparticles
To synthesize AgNPs, 5.0 ml of the phlomis plant extract was added into 45.0 ml of 0.01 M of silver nitrate solution at room temperature. The change from pale yellow and dark brown indicated the formation of colloidal silver nanoparticles.
Characterization of silver nanoparticles
UV–visible absorption analysis was carried out using a SUV–S2100 spectrophotometer. FT–IR spectra of silver nanoparticles were performed using a JASCO FTIR (680 plus, Japan) spectrometer with KBr pellet in the range of 4000–400 cm−1. The crystallaine nature of silver nanoparticles was investigated by XRD analysis. X–ray diffraction data of AgNPs were obtained using a Philips–X’Pert Pro MPD with Cu kα radiation (λ = 1.54 Å) in the 2θ range of 20° to 80°, and with a steps size of 0.02° at 40 kV and 30 mA. The morphology of the AgNPs was examined by TEM (cm30–Philphs). Furthermore, SEM (HITACHI S–4160) study was carried out to investigate the shape, size and the surface area of the AgNPs.
Antibacterial assay
The antibacterial activity of bio–synthesized AgNPs was tested against various positive and negative bacteria by the standard agare well diffusion method. To examine the antibacterial activity of biosynthesized AgNPs, Muller-Hinton agar plates were sterilized and allowed to solidify. After solidification, 30 µl of each bacterial suspension was inoculated on the petriplates by a strile glass rod. Then, 0.1 g of synthesized–AgNPs powder were dissolved in 1.0 ml (100 ppm) autoclaved distillated water to provide the suspension of AgNPs. Sterilized paper disks (diameter 6.4 mm) were impregnated with 30 μl of suspension and placed on Muller Hinton agar plates. The negative (distilled water) and positive (AgNO3) controls, and the phlomis leaf extract were also employed for the antibacterial assay. The plates were incubated at 37 °C for 24 h. After the incubation period, the zones of inhibitions were observed around the discs. Antibacterial activity was investigated by measuring the diameter of the zones of inhibition after using the plant extract.
Results and discussion
UV–Vis spectral studies and FT–IR analysis
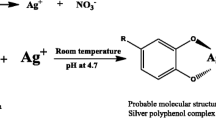
The phlomis plant extract was employed for the green synthesis of AgNPs. After the addition of the plant leaf extract to the silver nitrate solution, it was observed that the color of the reaction mixture was gradually changed from light yellow to dark brown, indicating the formation of silver nanoparticles. UV–Vis absorption spectroscopy is an important method to detect the formation and stability of metal NPs in the reaction mixture. Figure 1 shows the UV–Visible spectra recorded at different times of reaction. No change in absorbance was observed after 30 min, indicating the complete conversion of Ag+ to Ag. In this work, the UV–vis spectra of silver nanoparticles synthesized displayed a strong broad peak around 440 nm due to the formation of AgNPs. This peak corresponded to the surface plasmon resonance of the synthesized AgNPs [29]. UV–VIS absorption measurements for silver NPs further were confiris in the range of 450–500 nm [2].
To determine the possible biomolecules and functional groups involved in reduction, capping and efficient stabilization of newly synthesized Ag nanoparticles, FTIR spectroscopy was employed. The FTIR spectrum of stabilized silver nanoparticles is depicted in Fig. 2. The spectra showed absorption bands at 3419, 1749, 2927, 1625, 1383, 1235, 1069, 832 and 602 cm−1. The strong peaks at 3419 cm−1 corresponded to –OH stretching due to phenolic compounds present in the phlomis leaf extract. The band at 2927 cm−1 was attributed to alkane C–H stretching vibration. C=O stretching possibly due to the presence of carbonil group. The peak at 1625 cm−1 corresponded to C=C stretching vibration of aromatic rings. The peak at 1383 cm−1 was corresponded to C–H bending. Further, peaks assigned at 1235 and 1069 cm−1 were attributed to C–N stretching possibly due to the presence of amines group. Another intense band at 832 cm−1 was characteristic of the aromatic ring [30–33].
The precipitation stage during the reduction process usually involves fast chemical reactions and nucleation kinetics, thus the type of reducer a significant factor in the particle size distribution of the product [34]. The results previously reported indicated that the phlomis leaf extract containing glycosides such as flavonoids, iridoids, diterpenoids, phenylpropanoids, triterpenoids and other phenolic compounds possibility contributed to the process of nanoparticle synthesis [15]. Furthermore, based on the above results, it was clear that the functional groups like –OH (hydroxyl), –C=O (Carbonil) and C–N (amine) present in the leaf extract were involved in synthesis of AgNPs.
XRD analysis
The X-ray diffraction analyses were carried out to determine the known phase of the silver nanoparticles. Figure 3 demonstrates the XRD pattern of the dried synthesized Ag nanoparticles by the phlomis extract. The spectrum exhibited five distinct separate peaks at 2θ = 38.10°, 44.37°, 64.17°, 77.57° and 81.67° that could be indexed to (111) (200), (220), (311) and (222) planes, respectively. The diffraction peaks data obtained were in accordance with the reports of FCC structure from Joint Committee Powder Diffraction Standards (JCPDS) file No. 04–0783. The mean grain crystalline size of green synthesized AgNPs was calculated by employing Debye–Scherrer formula.
where D is the average crystalline diameter size (Å), K is a constant (0.9), ‘λ’ is the X-ray wavelength used (λ = 1.54 Å), ‘β’ is the angular line width at the half maximum of diffraction (radians) and ‘θ’ is the braggs angle (degrees) [11]. The average grain crystalline size of AgNPs was estimated to be approximately 27 nm. A small number of unassigned peaks (marked with stars) were also recorded that might be due to the crystallization of bioorganic phases present in phlomis extract on the surface of the silver nanoparticles. Similar results were also obtained for AgNPs synthesized using the beetroot extract and Ixora coccinea leaves extract [35, 36].
SEM and TEM analysis
SEM technique was employed to determine the surface morphology and the topography of synthesized silver nanoparticles. Figure 4 shows the size of silver nanoparticles from 19 to 30 nm, with an average size 25 nm. SEM image exhibited that the biosynthesized silver nanoparticles were mostly spherical in shape. The shape and size of the biosynthesized AgNPs were further analyzed by TEM. TEM image (Fig. 5) demonstrated that the most AgNPs were obviously spherical in shape and well dispersed, with an average size around 25 nm. The obtained results from TEM image were in a good agreement with the SEM data.
Antibacterial activity
The antibacterial activity of biosynthesized silver nanoparticles, AgNO3, phlomis leaf extract and distilled water was studied against Gram-positive (S. aureus and B. cereus) and Gram-negative (S. typhimurium and E. coli) bacteria using the agar well diffusion assay, and the zone of inhibition was tabulated as shown in Table 1 and Fig. 6. The synthesized AgNPs displayed efficient antibacterial activity against both Gram-negative and Gram-positive bacteria. The silver nanoparticles synthesized by phlomis leaf extracts showed the maximum zone of inhibition around 15 mm for S. typhimurium and E. coli, which were followed by S. aureus (14.7 mm) and B. cereus (12.1 mm). On the other hand, the negative control (distilled water) and the leaf extract did not exhibit any zone of inhibition. The positive control (AgNO3) displayed antimicrobial activity against all tested microorganisms, Gram-negative bacteria, E. coli (11.3 mm) and S. typhimurium (9.5 mm), and Gram-positive bacteria, S. aureus and B. cereus (9.4 mm).
The mechanisms of efficient antibacterial activity by silver nanoparticles against various pathogenic bacteria, are still unclear and required further investigation [30, 35]. AgNPs might have been attached to the surface of the cell membrane of microorganisms, leading to the disturbance of its functions like permeability and respiration. It is obvious, therefore, that the binding of particles to the microorganism depends on the surface area available for intraction. In general, small nanoparticles have a larger surface area for intraction with bacteria, as compared to that of bigger particles, due to greater antibacterial activity [35, 37, 38]. In our results, the Gram-positive bacteria showed the lower zone of inhibition, as compared to Gram-negative bacteria. This could be due to the cell wall of Gram-positive bacteria composed of a rigid thicker multiple layer of peptidoglycan, as it prevented the nanoparticles from entering into cell wall [39].
Conclusion
In the present study, we showed, for the first time, the biosynthesis of stable and nearly spherical silver nanoparticles using the phlomis plant extract as a reducing and capping agent. The biosynthesized nanoparticles presented strong antimicrobial activity against Gram-positive (S. aureus and B. cereus) and Gram-negative (S. typhimurium and E. coli) bacteria. Also, we confirmed the formation of silver nanoparticles using the phlomis plant extract from UV–Vis, XRD, SEM, and FTIR. UV–Vis peak was observed for AgNPs at 450 nm. The synthesized AgNPs were found to have a crystalline structure as investigated by XRD method. The particle size of the silver nanoparticles ranged in size from 19 to 30 nm, with an average size of 25 nm. Finally, the green synthesis of silver nanoparticles using plant material was found to be the most eco–friendly and conventional method, in comparison to chemical and physical synthesis methods.
References
Allafchian, A.R., Majidian, Z., Ielbeigi, V., Tabrizchi, M.: A novel method for the determination of three volatile organic compounds in exhaled breath by solid-phase microextraction–ion mobility spectrometry. Anal. Bioanal. Chem. 408, 839–847 (2016)
Mittal, A.K., Chisti, Y., Banerjee, U.C.: Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 31, 346–356 (2013)
Muthoosamy, K., Bai, R.G., Abubakar, I.B., Sudheer, S.M., Lim, H.N., Loh, H.S., Huang, N.M., Chia, ChH, Manickam, S.: Exceedingly biocompatible and thin-layered reduced graphene oxide nanosheets using an eco-friendly mushroom extract strategy. Int. J. Nanomedicine. 10, 1505–1519 (2015)
Ng, C.M., Chen, P.C., Manickam, S.: Green high-gravitational synthesis of silver nanoparticles using a rotating packed bed reactor (RPBR). Ind. Eng. Chem. Res. 51, 5375–5381 (2012)
Kuppusamy, P., Ichwan, S.J., Parine, N.R., Yusoff, M.M., Maniam, G.P., Govindan, N.: Intracellular biosynthesis of Au and Ag nanoparticles using ethanolic extract of Brassica oleracea L. and studies on their physicochemical and biological properties. J. Environ. Sci. 29, 151–157 (2015)
Prakash, P., Gnanaprakasam, P., Emmanuel, R., Arokiyaraj, S., Saravanan, M.: Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf. B 108, 255–259 (2013)
Orbaek, A.W., McHale, M.M., Barron, A.R.: Synthesis and characterization of silver nanoparticles for an undergraduate laboratory. J. Chem. Edu. 92, 339–344 (2014)
Bar, H., Bhui, D.K., Sahoo, G.P., Sarkar, P., Pyne, S., Misra, A.: Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf. A 348, 212–216 (2009)
Otari, S., Patil, R., Nadaf, N., Ghosh, S., Pawar, S.: Green biosynthesis of silver nanoparticles from an actinobacteria Rhodococcus sp. Mater. Lett. 72, 92–94 (2012)
Allafchian, A.R., Bahramian, H., Jalali, S.A.H., Ahmadvand, H.: Synthesis, characterization and antibacterial effect of new magnetically core–shell nanocomposites. J. Magn. Magn. Mater. 394, 318–324 (2015)
Allafchian, A.R., Jalali, S.A.H.: Synthesis, characterization and antibacterial effect of poly (acrylonitrile/maleic acid)–silver nanocomposite. J. Taiwan Inst. Chem. Eng. 57, 154–159 (2015)
Kalishwaralal, K., Deepak, V., Pandian, S.R.K., Kottaisamy, M., BarathManiKanth, S., Kartikeyan, B., et al.: Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surf. B 77, 257–262 (2010)
Allafchian, A., Jalali, S.A.H., Bahramian, H., Ahmadvand, H.: Preparation, characterization, and antibacterial activity of NiFe2O4/PAMA/Ag–TiO2 nanocomposite. J. Magn. Magn. Mater. 404, 14–20 (2016)
Sarikurkcu, C., Uren, M.C., Tepe, B., Cengiz, M., Kocak, M.S.: Phenolic content, enzyme inhibitory and antioxidative activity potentials of Phlomis nissolii and P. pungens var. pungens. Ind. Crop. Prod. 62, 333–340 (2014)
Sarkhail, P., Rahmanipour, S., Fadyevatan, S., Mohammadirad, A., Dehghan, G., Amin, G., et al.: Antidiabetic effect of Phlomis anisodonta: effects on hepatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Pharmacol. Res. 56, 261–266 (2007)
Huang, J., Li, Q., Sun, D., Lu, Y., Su, Y., Yang, X., et al.: Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotech. 18, 105104 (2007)
Dwivedi, A.D., Gopal, K.: Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf. A 369, 27–33 (2010)
Yilmaz, M., Turkdemir, H., Kilic, M.A., Bayram, E., Cicek, A., Mete, A., et al.: Biosynthesis of silver nanoparticles using leaves of Stevia rebaudiana. Mater. Chem. Phys. 130, 1195–1202 (2011)
Philip, D., Unni, C., Aromal, S.A., Vidhu, V.: Murraya koenigii leaf–assisted rapid green synthesis of silver and gold nanoparticles. Spectrochim. Acta A 78, 899–904 (2011)
Vivek, R., Thangam, R., Muthuchelian, K., Gunasekaran, P., Kaveri, K., Kannan, S.: Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF–7 cells. Process Biochem. 47, 2405–2410 (2012)
Thirunavoukkarasu, M., Balaji, U., Behera, S., Panda, P., Mishra, B.: Biosynthesis of silver nanoparticle from leaf extract of Desmodium gangeticum (L.) DC. and its biomedical potential. Spectrochim. Acta A 116, 424–427 (2013)
Khan, M., Khan, M., Adil, S.F., Tahir, M.N., Tremel, W., Alkhathlan, H.Z., et al.: Green synthesis of silver nanoparticles mediated by Pulicaria glutinosa extract. Int. J. nanomedicine. 8, 1507–1516 (2013)
Notriawan, D., Angasa, E., Suharto, T.E., Hendri, J., Nishina, Y.: Green synthesis of silver nanoparticles using aqueous rinds extract of Brucea javanica (L.) Merr at ambient temperature. Mater. Lett. 97, 181–183 (2013)
Jagtap, U.B., Bapat, V.A.: Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. seed extract and its antibacterial activity. Ind. Crop. Prod. 46, 132–137 (2013)
Jeeva, K., Thiyagarajan, M., Elangovan, V., Geetha, N., Venkatachalam, P.: Caesalpinia coriaria leaf extracts mediated biosynthesis of metallic silver nanoparticles and their antibacterial activity against clinically isolated pathogens. Ind. Crop. Prod. 52, 714–720 (2014)
Ashokkumar, S., Ravi, S., Kathiravan, V., Velmurugan, S.: Synthesis of silver nanoparticles using A. indicum leaf extract and their antibacterial activity. Spectrochim. Acta A 134, 34–39 (2015)
Manikandan, R., Manikandan, B., Raman, T., Arunagirinathan, K., Prabhu, N.M., Basu, M.J., et al.: Biosynthesis of silver nanoparticles using ethanolic petals extract of Rosa indica and characterization of its antibacterial, anticancer and anti–inflammatory activities. Spectrochim. Acta A 138, 120–129 (2015)
Rajaram, K., Aiswarya, D., Sureshkumar, P.: Green synthesis of silver nanoparticle using Tephrosia tinctoria and its antidiabetic activity. Mater. Lett. 138, 251–254 (2015)
Velusamy, P., Das, J., Pachaiappan, R., Vaseeharan, B., Pandian, K.: Greener approach for synthesis of antibacterial silver nanoparticles using aqueous solution of neem gum (Azadirachta indica L.). Ind. Crop. Prod. 66, 103–109 (2015)
Ahmed, M.J., Murtaza, G., Mehmood, A., Bhatti, T.M.: Green synthesis of silver nanoparticles using leaves extract of Skimmia laureola: characterization and antibacterial activity. Mater. Lett. 153, 10–13 (2015)
Joseph, S., Mathew, B.: Microwave–assisted green synthesis of silver nanoparticles and the study on catalytic activity in the degradation of dyes. J. Mol. Liq. 204, 184–191 (2015)
Kumar, P.V., Pammi, S., Kollu, P., Satyanarayana, K., Shameem, U.: Green synthesis and characterization of silver nanoparticles using Boerhaavia diffusa plant extract and their anti bacterial activity. Ind. Crop. Prod. 52, 562–566 (2014)
Kumar, S., Singh, M., Halder, D., Mitra, A.: Mechanistic study of antibacterial activity of biologically synthesized silver nanocolloids. Colloids Surf. A 449, 82–86 (2014)
Skoog, D., West, D., Holler, F., Crouch, S.: Fundamentals of analytical chemistry. 9th edn. Brooks Cole (2013)
Bindhu, M., Umadevi, M.: Silver and gold nanoparticles for sensor and antibacterial applications. Spectrochim. Acta A. 128, 37–45 (2014)
Karuppiah, M., Rajmohan, R.: Green synthesis of silver nanoparticles using Ixora coccinea leaves extract. Mater. Lett. 97, 141–143 (2013)
Mata, R., Bhaskaran, A., Sadras, S.R.: Green–synthesized gold nanoparticles from Plumeria alba flower extract to augment catalytic degradation of organic dyes and inhibit bacterial growth. Particuology. (2015). doi:10.1016/j.partic.2014.12.014
Naraginti, S., Sivakumar, A.: Eco–friendly synthesis of silver and gold nanoparticles with enhanced bactericidal activity and study of silver catalyzed reduction of 4–nitrophenol. Spectrochim. Acta A. 128, 357–362 (2014)
Ahmed, S., Ahmad, M., Swami, B.L., Ikram, S.: A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J. Adv. Res. (2015). doi:10.1016/j.jare.2015.02.007
Acknowledgements
The authors wish to thank Isfahan University of Technology (IUT) Research Council, Center of Excellency in Applied Nanotechnology and the Iranian Nanotechnology Council for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Allafchian, A.R., Mirahmadi-Zare, S.Z., Jalali, S.A.H. et al. Green synthesis of silver nanoparticles using phlomis leaf extract and investigation of their antibacterial activity. J Nanostruct Chem 6, 129–135 (2016). https://doi.org/10.1007/s40097-016-0187-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-016-0187-0