Abstract
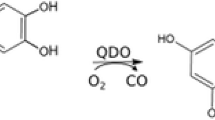
Homoprotocatechuate (HPCA) dioxygenases are enzymes that take part in the catabolism of aromatic compounds in the environment. They use molecular oxygen to perform the ring cleavage of ortho-dihydroxylated aromatic compounds. A theoretical investigation of the catalytic cycle for HPCA 2,3-dioxygenase isolated from Brevibacterium fuscum (Bf 2,3-HPCD) was performed using hybrid DFT with the B3LYP functional, and a reaction mechanism is suggested. Models of different sizes were built from the crystal structure of the enzyme and were used in the search for intermediates and transition states. It was found that the enzyme follows a reaction pathway similar to that for other non-heme iron dioxygenases, and for the manganese-dependent analog MndD, although with different energetics. The computational results suggest that the rate-limiting step for the whole reaction of Bf 2,3-HPCD is the protonation of the activated oxygen, with an energy barrier of 17.4 kcal/mol, in good agreement with the experimental value of 16 kcal/mol obtained from the overall rate of the reaction. Surprisingly, a very low barrier was found for the O–O bond cleavage step, 11.3 kcal/mol, as compared to 21.8 kcal/mol for MndD (sextet spin state). This result motivated additional studies of the manganese-dependent enzyme. Different spin coupling between the unpaired electrons on the metal and on the evolving substrate radical was observed for the two enzymes, and therefore the quartet spin state potential energy surface of the MndD reaction was studied. The calculations show a crossing between the sextet and the quartet surfaces, and it was concluded that a spin transition occurs and determines a barrier of 14.4 kcal/mol for the O–O bond cleavage, which is found to be the rate-limiting step in MndD. Thus the two 83% identical enzymes, using different metal ions as co-factors, were found to have similar activation energies (in agreement with experiment), but different rate-limiting steps.










Similar content being viewed by others
References
Ornston LN, Stanier RY (1966) J Biol Chem 241:3776–3786
Solomon EI, Brunold TC, Davis MI, Kemsley JN, Lee SK, Lehnert N, Neese F, Skulan AJ, Yang YS, Zhou J (2000) Chem Rev 100:235–350
Que L Jr, Ho RYN (1996) Chem Rev 96:2607–2624
Que L Jr (2000) Nat Struct Biol 7:182–184
Dagley S (1977) Surv Prog Chem 8:121–170
Broderick JB (1999) Essays Biochem 34:173–189
Lipscomb JD, Orville AM (1992) Met Ions Biol Syst 28:243–298
Vetting MW, Wackett LP, Que L Jr, Lipscomb JD, Ohlendorf DH (2004) J. Bacteriol 186:1945–1958
Bassan A, Borowski T, Siegbahn PEM (2004) Dalton Trans 20:3153–3162
Whiting AK, Boldt YR, Hendrich LP, Wackett LP, Que L Jr (1996) Biochemistry 35:160–170
Groce SL, Lipscomb JD (2003) J Am Chem Soc 125:11780–11781
Sato N, Uragami Y, Nishizaki T, Takahashi Y, Sazaki G, Sugimoto K, Nokana T, Masai E, Fukuda M, Senda T (2002) J Mol Biol 321:621–636
Arciero DM, Orville AM, Lipscomb JD (1985) J Biol Chem 260:14035–14044
Bugg TDH, Lin G (2001) Chem Commun 11:941–952
Miller MA, Lipscomb JD (1996) J Biol Chem 271:5524–5535
Shu L, Chiou YM, Orville AM, Miller MA, Lipscomb JD, Que L Jr (1995) Biochemistry 34:6649–6659
Groce SL, Lipscomb JD (2005) Biochemistry 44:7175–7188
Emerson J, Wagner ML, Reynolds MF, Que L Jr, Sadowsky MJ, Wacket LP (2005) J Biol Inorg Chem 10:751–760
Kovaleva ED, Lipscomb JD (2007) Science 316:453–457
Kovaleva ED, Neibergall MB, Chakrabarty S, Lipscomb JD (2007) Acc Chem Res 40:475–483
Georgiev V, Borowski T, Siegbahn PEM (2006) J Biol Inorg Chem 11:571–585
Schrödinger Inc. (2000) JAGUAR 5.5. Portland, Oregon
Frisch MJ, Trucks GW, Schlegel HB, et al. (2004) Gaussian 03, Revision D.01. Gaussian, Inc., Wallingford, CT
Harvey JN, Aschi M, Schwarz H, Koch W (1998) Theor Chem Acc 99:95–99
Mouesca J-M, Chen JC, Noodleman L, Bashford D, Case DA (1994) J Am Chem Soc 116:11898–11914
Noodleman L, Case DA (1992) Adv Inorg Chem 38:423–470
Himo F (2005) Biochim Biophys Acta–Bioenerg 1707:24–33
Bassan A, Blomberg MRE, Siegbahn PEM (2004) J Biol Inorg Chem 9:439–452
Groce SL, Miller-Rodeberg MA, Lipscomb JD (2004) Biochemistry 43:15141–15153
Arciero DM, Lipscomb JD, Huynh BH, Kent TA, MÃnck E (1983) J Biol Chem 258:14981–14991
Shu L, Chiou YM, Orville AM, Miller MA, Lipscomb JD, Que L Jr (1995) Biochemistry 34:6649–6659
Mabrouk PA, Orville AM, Lipscomb JD, Solomon EI (1991) J Am Chem Soc 113:4053–4061
Vaillancourt FH, Barbosa CJ, Spiro TG, Bolin JT, Blades MW, Turner RFB, Eltis LD (2002) J Am Chem Soc 124:2485–2496
Wirstam M, Lippard SJ, Friesner RA (2003) J Am Chem Soc 125:3980–3987
Lundberg M, Morokuma K (2007) J Phys Chem 111:9380–9389
Bassan A, Blomberg, MRE, Siegbahn PEM (2003) Chem Eur J 9:106–115
Siegbahn PEM, Haeffner F (2004) J Am Chem Soc 126:8919–8932
Borowski T, Georgiev V, Siegbahn PEM (2005) J Am Chem Soc 127:17303–17314
Harvey JN, Poli R, Smith KM (2003) Coord Chem Rev 238–239:347–361
Acknowledgments
T.B. acknowledges the support from the Polish State Committee for Scientific Research (Grant N204 173 31/3823).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Georgiev, V., Borowski, T., Blomberg, M.R.A. et al. A comparison of the reaction mechanisms of iron- and manganese-containing 2,3-HPCD: an important spin transition for manganese. J Biol Inorg Chem 13, 929–940 (2008). https://doi.org/10.1007/s00775-008-0380-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-008-0380-9