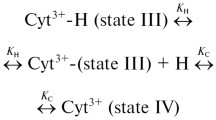Abstract
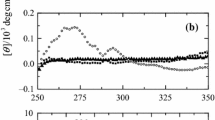
A relation between pH-induced conformational transitions of horse heart ferricytochrome c and the kinetics of external ligand coordination to heme iron was investigated by optical spectroscopy, circular dichroism and viscometry. The dependencies of both the association, k a, and dissociation rate constants of cyanide binding on pH were determined from kinetic measurements. The association rate constant exhibits a bell-shaped form of dependence on pH in the region where this protein unfolds. The maximum of the dependence of k a on pH is found to be coincident with the pK values of conformational transitions of ferricytochrome c in solutions with both low and high ionic strengths. This observation is explained in terms of ferricytochrome c unfolding, which is characterized by two processes: the gradual opening of the heme crevice accompanied by the detachment of the axial Met80 and its replacement with a water molecule. The former process enhances the rate, whereas the latter results in the inhibition of the rate of cyanide binding.








Similar content being viewed by others
References
Varhač R, Antalík M (2004) Biochemistry 43:3564–3569
Goto Y, Calciano LJ, Fink AL (1990) Proc Natl Acad Sci USA 87:573–577
Shaw RW, Hartzell CR (1976) Biochemistry 15:1909–1914
Berka V, Vygodina T, Musatov A, Nicholls P, Konstantinov AA (1993) FEBS Lett 315:237–241
Panda M, Robinson NC (1995) Biochemistry 34:10009–10018
Fabian M, Palmer G (1995) Biochemistry 34:1534–1540
Klapper MH, Uchida H (1971) J Biol Chem 246:6849–6854
Dou Y, Olson JS, Wilkinson AJ, Ikeda-Saito M (1996) Biochemistry 35:7107–7113
Bidwai A, Witt M, Foshay M, Vitello LB, Satterlee JD, Erman JE (2003) Biochemistry 42:10764–10771
Goldsack DE, Eberlein WS, Alberty RA (1966) J Biol Chem 241:2653–2660
Ver Ploeg DA, Alberty RA (1968) J Biol Chem 243:435–440
Smerdon SJ, Krzywda S, Brzozowski AM, Davies GJ, Wilkinson AJ, Brancaccio A, Cutruzzolá F, Allocatelli CT, Brunori M, Li T, Brantley RE Jr, Carver TE, Eich RF, Singleton E, Olson JS (1995) Biochemistry 34:8715–8725
Olson JS, Phillips GN Jr (1997) J Biol Inorg Chem 2:544–552
Brancaccio A, Cutruzzolá F, Allocatelli CT, Brunori M, Smerdon SJ, Wilkinson AJ, Dou Y, Keenan D, Ikeda-Saito M, Brantley RE Jr, Olson JS (1994) J Biol Chem 269:13843–13853
Foshay MC, Vitello LB, Erman JE (2004) Biochemistry 43:5065–5072
Dumortier C, Holt JM, Meyer TE, Cusanovich MA (1998) J Biol Chem 273:25647–25653
Dumortier C, Meyer TE, Cusanovich MA (1999) Arch Biochem Biophys 371:142–148
Tomášková N, Varhač R, Žoldák G, Olekšáková L, Sedláková D, Sedlák E (2007) J Biol Inorg Chem 12:257–266
Mintorovitch J, Satterlee JD (1988) Biochemistry 27:8045–8050
Motie M, Kassner RJ, Meyer TE, Cusanovich MA (1990) Biochemistry 29:1932–1936
George P, Tsou CL (1952) Biochem J 50:440–448
Job D, Zeba B, Puppo A, Rigaud J (1980) Eur J Biochem 107:491–500
Erman JE (1974) Biochemistry 13:39–44
Ellis WD, Dunford HB (1968) Biochemistry 7:2054–2062
Morishima I, Inubushi T (1978) J Am Chem Soc 100:3568–3574
Yoshikawa S, O’Keeffe DH, Caughey WS (1985) J Biol Chem 260:3518–3528
Behere DV, Gonzales-Vergara E, Goff HM (1985) Biochim Biophys Acta 832:319–325
Yao Y, Qian C, Ye K, Wang J, Bai Z, Tang W (2002) J Biol Inorg Chem 7:539–547
Thanabal V, de Ropp JS, La Mar GN (1988) J Am Chem Soc 110:3027–3035
Satterlee JD, Erman JE (1983) J Biol Chem 258:1050–1056
Milani M, Ouellet Y, Ouellet H, Guertin M, Boffi A, Antonini G, Bocedi A, Mattu M, Bolognesi M, Ascenzi P (2004) Biochemistry 43:5213–5221
Poulos TL, Freer ST, Alden RA, Xuong NH, Edwards SL, Hamlin RC, Kraut J (1978) J Biol Chem 253:3730–3735
Mintorovitch J, van Pelt D, Satterlee JD (1989) Biochemistry 28:6099–6104
Ikeda-Saito M (1987) Biochemistry 26:4344–4349
Bánó M, Strhársky I, Hrmo I (2003) Rev Sci Instrum 74:4788–4793
Sutin N, Yandell JK (1972) J Biol Chem 247:6932–6936
Shechter E, Saludjian P (1967) Biopolymers 5:788–790
Goto Y, Takahashi N, Fink AL (1990) Biochemistry 29:3480–3488
Greenwood C, Wilson MT (1971) Eur J Biochem 22:5–10
Stellwagen E (1968) Biochemistry 7:2893–2898
Myer YP, Saturno AF (1990) J Protein Chem 9:379–387
Myer YP, Saturno AF (1991) J Protein Chem 10:481–494
Sedlák E, Antalík M (1999) Biochim Biophys Acta 1434:347–355
Theorell H, Åkesson Å (1941) J Am Chem Soc 63:1812–1818
Lanir A, Yu N-T, Felton RH (1979) Biochemistry 18:1656–1660
Myer YP, Srivastava RB, Kumar S, Raghavendra K (1983) J Protein Chem 2:13–42
Oellerich S, Wackerbarth H, Hildebrandt P (2002) J Phys Chem B 106:6566–6580
Santucci R, Bongiovanni C, Mei G, Ferri T, Polizio F, Desideri A (2000) Biochemistry 39:12632–12638
Jordan T, Eads JC, Spiro TG (1995) Protein Sci 4:716–728
Indiani C, de Sanctis G, Neri F, Santos H, Smulevich G, Coletta M (2000) Biochemistry 39:8234–8242
Babul J, Stellwagen E (1971) Biopolymers 10:2359–2361
Giacometti GM, Ascenzi P, Brunori M, Rigatti G, Giacometti G, Bolognesi M (1981) J Mol Biol 151:315–319
Stryer L, Kendrew JC, Watson HC (1964) J Mol Biol 8:96–104
Behere DV, Ales DC, Goff HM (1986) Biochim Biophys Acta 871:285–292
Bay Y, Sosnick TR, Mayne L, Englander SW (1995) Science 269:192–197
Acknowledgments
This work was supported by research grants 2/6167/26 and 1/3252/06 from the Slovak Grant Agency. We thank Dr. Marián Fabian for his editorial help in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Varhač, R., Antalík, M. Correlation of acid-induced conformational transition of ferricytochrome c with cyanide binding kinetics. J Biol Inorg Chem 13, 713–721 (2008). https://doi.org/10.1007/s00775-008-0357-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-008-0357-8